International Journal of
eISSN: 2381-1803


Research Article Volume 17 Issue 3
1Biotechnology and Genetic Engineering Discipline, Khulna University, Bangladesh
2Pharmacy Discipline, Khulna University, Bangladesh
3New Mexico Institute of Mining and Technology, USA
Correspondence: Md. Emdadul Islam, Biotechnology and Genetic Engineering Discipline, Khulna University, Khulna-9208, Bangladesh
Received: June 24, 2024 | Published: July 9, 2024
Citation: Emdadul I, Kazi DI, Morsaline B, et al. 15-lipoxygenase and serine protease inhibitory activities of Heritiera fomes (buch. -ham), a mangrove plant of the Sundarbans. Int J Complement Alt Med. 2024;17(3):156-162. DOI: 10.15406/ijcam.2024.17.00697
15-Lipoxygenase (15-Lox) oxidizes fatty acid, thereby producing inflammatory mediators which cause diverse inflammatory diseases such as atherosclerosis, asthma, cancer etc. Serine proteases are enzymes that activate tissue factor VII by proteolytic cleavage, eventually activating blood coagulation pathway. Antioxidants play role in 15-Lox and serine protease inhibition to dampen elevation of cytokine levels, thereby function as anti-inflammation and anticoagulation agent. In this study, we examined 15-Lox and serine protease inhibitory activities of the ethanolic bark and leaf crude extract of Heritiera fomes. The crude bark extract (HFB) exhibited 15-Lox and serine protease inhibition with IC50 117.14 and 409.61 μg/ml, respectively. Chromatographically separated active fraction HFBPF1 demonstrated highest 15-Lox and serine protease inhibitory activity with IC50 62.68 and 72.62 μg/ml, respectively. The antioxidant activity and total flavonoid content were substantially increased in the chromatographic fractions compared to the crude extracts. The LC-MS/MS analysis of bark and leaf active fractions revealed the presence of 2,4,6-octatrienal, apigenin 7 (4˝-Z-p-coumerylglucoside), brosimacutin B, gambrin B2, kandelin A1 and tetraneurin A which we report for the first time in H. fomes. Therefore, we conclude the significant augmentation in the 15-Lox and serine protease inhibition activity of the fractions which could be exerted by the enriched flavonoid content.
Keywords: 15-Lipoxygenase, serine protease, antioxidant, anti-inflammation, anti-coagulation, H. fomes
15-Lipooxygenase (15-Lox) is a non-heme iron containing enzyme belonging to lipooxygenase family that catalyze the dioxygenation of polyunsaturated fatty acids (e.g. arachidinic acid, linoleic acid etc) and produce 15- hydroperoxyecosatetraenoic acids (15-HPETE) which is further reduced to corresponding hydroxyeicosatetraenoic acid (15-HETEs) or converted to other types of eicosanoids such as leucotriens. Reactive oxygen species (ROS) propagate inflammation by activation of enzymes such as 15-lipoxygenase from inflammatory cells. 15-lipoxygenase The ROS induced activation of 15-lipoxygenase causes the onset of many diseases including atherosclerosis, diabetes, arthritis, hyper tension, inflammatory bowel disease and peripheral atherogenesis and cancer.1,2 There is contrasting effect of 15-Lox on the development of inflammatory diseases in different animal models or in human epidemiological studies due to both pro- and anti-atherogenic effects mediated via particular lipid metabolites.1
Inflammation also initiates clotting, decreases the activity of natural anticoagulant mechanisms and impairs the fibrinolytic system. Inflammatory cytokines are the major mediators involved in coagulation activation (Esmon, 2005).3 Serine proteases are enzymes that activate tissue factor VII by proteolytic cleavage, eventually activating blood coagulation pathway. Serine protease induced activation of clot factors thus causes in coagulation of blood in the arterial system resulting atherogenesis. Down regulation of anticoagulant pathways not only promotes thrombosis but also amplifies the inflammatory process.3
The inhibition of lipoxygenase-catalyzed lipid metabolism and serine protease-mediated activation of blood coagulation pathway is the preferred mode to combat the above-mentioned clinical conditions. Selective inhibitors of the 15-lipoxygenase and serine proteases are interesting areas of investigation and would be an important tool as well as a promising therapeutic target for treating a wide spectrum of human diseases.
Plants secondary metabolites present an attractive route to address the ever increasing need for new drugs, because of their novelty and structural diversity. Plants have been a good source of 15-Lox inhibitors4 as well as serine protease inhibitors.5 Mangroves are intertidal forest wetlands established at the interface between land and sea in tropical and subtropical region. They are well adapted in their extreme environmental conditions of high salinity, high temperature and anaerobic soils. Mangrove plants synthesize diversified secondary metabolites, some of which are unique and thus could be interesting for drug development process. Several recent initiatives have been reported on Lox inhibitors from different mangrove species.6
In the present study, we selected Heritiera fomes (Buch.Ham.) based on ethnomedicinal use in the treatment of inflammatory diseases and its reported inhibitory activity against 15-lipoxygenase.6,7 We examined the antioxidant activity by DPPH free radical scavenging assay, antioxidant metabolite content by determining total flavonoid content, 15-Lox inhibitory activity and serine protease inhibitory activity of the crude ethanolic extract of bark and leaf of Heritiera fomes. The crude extracts were then fractionated through column chromatography. 15-Lox inhibition activity, serine protease inhibition activity, antioxidant activity and flavonoid content of the active fractions were determined. The correlation among antioxidant content, antioxidant activity, 15-Lox inhibition activity and serine protease inhibition activity was studied. LC-MS/MS analysis was performed and the compounds present in the active fractions were identified.
Chemical and reagents
1, 1-Diphenyl-2-pycrylhydrazyl (DPPH), standards [quercetin, ascorbic acid, Nordihydroguaiaretic acid (NDGA), gallic acid] and linoleic acid were purchased from Sigma Aldrich (St. Louis, MO, USA). Other chemicals and solvents were purchased from Loba Chemie Pvt. Ltd (Mumbai, India). Solvents and all other reagents used in the study were of analytical grade.
Standard drugs: The standard drug Warfarin was collected from Square Pharma Ltd, Bangladesh and used for anti-coagulation activity study.
Collection of plant materials and extraction of secondary metabolites
Plant materials were collected from the forest Sundarbans with the permission of the appropriate authority of the forest department of the Sundarbans, Bangladesh. We strictly maintained the framework of the United Nations Convention on Biodiversity. Fresh leaves and bark of H. fomes were collected from the East Division of the Sundarbans mangrove forest, Bangladesh. After collection, it was identified at the Bangladesh National Herbarium, Dhaka (Accession no.: DACB 30324) and the voucher specimen was deposited. The bark and leaves were dried adequately under shade. The chopped and dried bark and leaves were then grinded into powder using a mechanical grinder. Cold extraction was used to prepare extract. About 400 gm of grinded powder were taken in an appropriate container and saturated in 900 ml of ethanol (99-100%). The container with its contents was kept for a time of 15 days with frequent stirring and shaking. The contents were filtered off with clear cotton plug to eliminate plant debrices. The extract was finally filtered through Whatman No.1 filter paper. The solvent (ethanol) was evaporated with electric fan at room temperature and finally the dried crude extract (yield value 12.5% and 9.6% for bark and leaf sample, respectively) was obtained.
Statistical analysis
Statistical analysis was performed using the software GraphPad Prism 6. All data were reported as means ± SD of three independent experiments and were analyzed by Two-way ANOVA followed by multiple comparison. For all comparisons, P<0.05 was considered statistically significant.
Bioassays
In vitro 15-Lox inhibition assay: The assay was based on the procedure according to Wangensteen et al.,8 (2006)with slight modification. The increase in absorbance at 234 nm was due to the formation of the product 13-hydroperoxyoctadecadienoic acid from the reaction of oxygen and linoleic acid. Substrate solution (134μM linoleic acid in ethanol), enzyme stock solution (soybean lipoxygenase 100000 U/ml) in 0.2 M borate buffer as well as five different concentrations (100μg/ml, 50 μg/ml, 25 μg/ml, 12.5 μg/ml and 6.25 μg/ml) of quercetin and plant extract solution was prepared with DMSO. Quercetin was used as standard inhibitor (positive control). The final concentration of enzyme in the assay mixture was used as 167 U/ml. Negative control was prepared with 25μl DMSO and 1 ml substrate solution with 975μl enzyme solution. Initially, 25μl quercetin/extract with 975μl enzyme solution was taken in cuvettes and incubated for 5 minutes at room temperature. Substrate solution (1ml) was then added in each cuvette except blank and negative control. Absorbance was recorded at 234 nm for 10 minutes at 1 minute interval. The absorbance was plotted against time and the rate of increase of absorbance was calculated. The percent inhibition of enzyme activity was calculated using the following equation:
Where, andare the slopes of the negative control and test sample respectively.
DPPH free radical scavenging assay: The free radical scavenging activity of the extract was estimated by DPPH assay.9 Aliquots of nine concentrations (1.57, 3.13, 6.25, 12.50, 25, 50, 100, 200 and 400 µg/ml) for plant extract and standard quercetin was made. Two ml of DPPH solution was applied on each test tube containing 1ml of serially diluted solution, and final volume of the mixture was 3ml. After 30 minutes of incubation at room temperature, absorbance of each test tube was determined at 517 nm. Inhibition of free radical scavenging activity was calculated using the equation:
IC50 value (µg/ml) is the inhibitory concentration at which DPPH free radicals are scavenged by 50%. A calibration curve of Quercetin was developed. IC50 value of the extracts and standard was calculated.
Determination of total flavonoid content: The method is based on formation of flavonoid-AlCl3 complex which is measured spectrophotometrically.10 Five different concentrations (50, 100, 150, 200 and 250µg/ml) of quercetin were prepared. The extracts were prepared at 100µg/ml concentration. Five ml distilled water and 0.3 ml of 5% NaNO2 was added to each tube followed by 0.6 ml of 10% AlCl3. Subsequently, 2 ml of 1M NaOH was added to the reaction mixture. The mixture was allowed to stand at room temperature for 5 minutes and the absorbance was measured at 510 nm and a standard calibration curve of quercetin was plotted. Values were expressed as microgram of quercetin equivalent per gram dry weight of plant extract.
Serine protease inhibition assay: This method is based on cleavage of the substrate casein by the serine protease enzyme trypsin.11,12 Cleavage of casein by trypsin is inhibited through inhibition of trypsin activity by the flavonoid present in the extract and the non degraded casein is measured at 280 nm. Quercetin was used as reference standard. Five different concentrations (400µg/ml, 200µg/ml, 100µg/ml, 50µg/ml and 25µg/ml) of sample extract and standard quercetin was prepared. 1ml of quercetin/extract was mixed with a preheated trypsin at 37°C for 15 minutes. Two ml of 1% casein was added with the reaction mixture and was allowed to stand for 30 minutes at 37°C. Then 3 ml of 5% trichloro acetic acid solution was added to stop the reaction. The absorbance of the supernatant was measured at 280 nm after centrifugation of the assay mixture at 12,000 rpm for 15 min. Percent inhibition of protease activity was calculated with the following equation
Chromatographic fractionation of crude extract: With the aim to enrich the flavonoid content, the crude ethanolic extracts were fractionated based on polarity of compound using C18 silica gel column. Ethanol extract of bark (20gm) and leaf of H. fomes (14gm) was subjected to column chromatography on silica gel packed and eluted with mixture of n-Hexane, ethyl acetate and methanol of increasing polarity to obtain fractions.
Thin Layer Chromatography (TLC) Analysis: TLC analysis was performed with chromatographically separated fractions and fractions containing similar banding pattern were pooled together. Antioxidant activity, flavonoid content, 15-Lox and serine protease inhibition activities were determined with the pooled fractions. LC-MS/MS analysis of the bioactive fractions was performed for identification of compound.
LC-MS/MS analysis: C18 reverse phase column of 1.8μm pore size and 2.1×50 mm diameter, 0.05% formic acid in water (solvent A), 0.05% formic acid in acetonitrile (solvent B), flow rate of 0.2 ml/min with positive ESI mode was used. Compound spectrum, MS spectrum, MS/MS spectrum was generated with MS/MS scan using Agilent Technology. LC-MS/MS data were analyzed automatically with the inbuilt software of Agilent Technology system. The name and the structure of the present compound were deduced with LC-MS/MS analysis.
15-Lox inhibition assay
The 15-Lox inhibitory activity of the ethanolic bark and leaf crude extract of H. fomes was investigated and the results are shown in Figure1. A concentration dependent effect on 15-Lox inhibition was observed in both bark (HFB) and leaf (HFL) crude extract (Figure 1a). The IC50 value of bark and leaf crude extract was 117.14μg/ml and 137.49μg/ml, respectively as compared to standard quercetin 54.39μg/ml (Figure 1b).
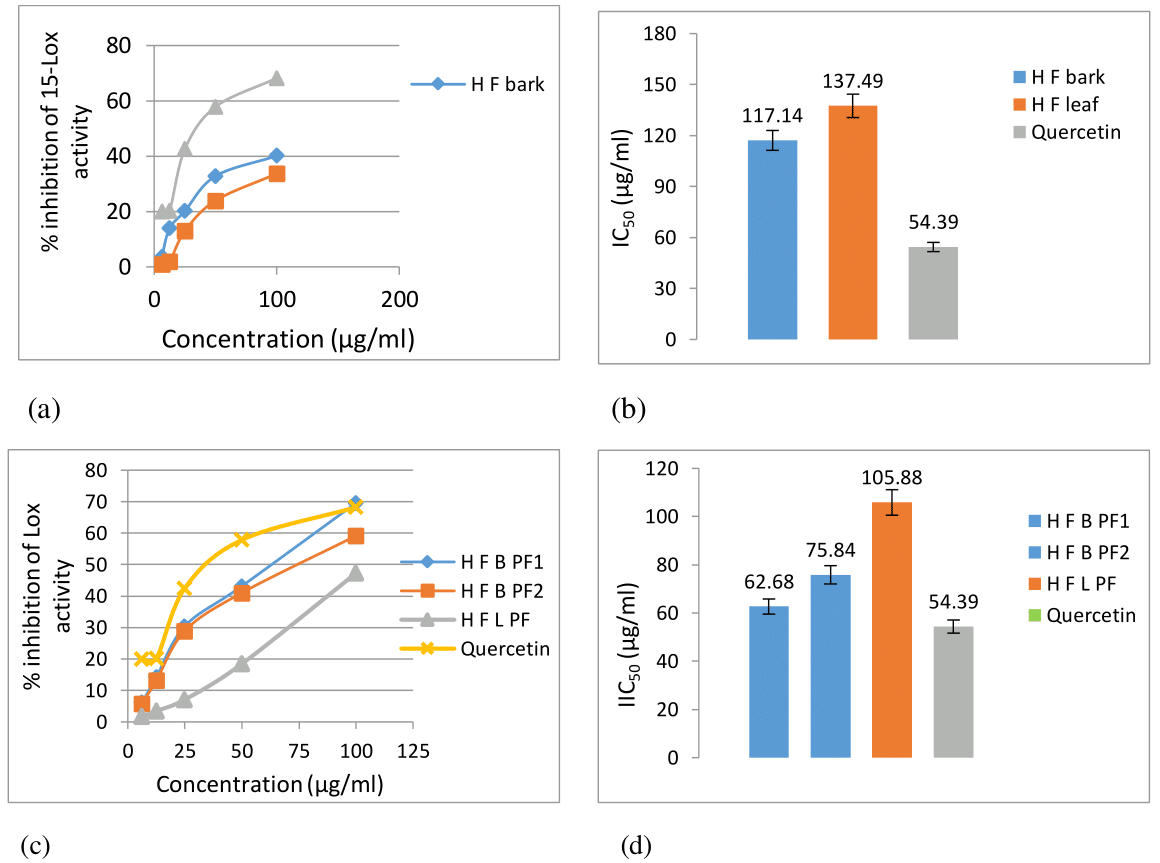
Figure 1 15-Lox inhibitory activity of ethanolic bark and leaf extracts of Heritiera fomes: (a) % inhibition of 15-Lox activity of bark and leaf crude extracts and standard quercetin; (b) IC50 values of bark and leaf crude extracts and standard ; (c) % inhibition of 15-Lox activity of chromatographically fractionated extracts; (d) ) IC50 values of b chromatographically fractionated extracts of bark and leaf and standard.
HFBPF1, H fomes bark pooled fraction 1, HFBPF2, H fomes bark pooled fraction 2, HFLPF, H fomes leaf pooled fraction.
The TLC analysis of chromatographically separated active fractions finally revealed two active fractions from bark (HFBPF1 and HFBPF2) and one active fraction (HFLPF) from leaf extract having 15-Lox inhibitory activity (Figure 1c). Increased 15-Lox inhibitory activity (IC50 value of HFBPF1: 62.68, HFBPF2: 75.84 and HFLPF: 105.88 µg/ml) was observed in the fractions of both bark and leaf extracts (Figure 1d).
Two-way ANOVA analysis revealed significant differences between control and treatment in all tested crude and purified fractions of both bark and leaf sample extract and therefore, the 15-Lox inhibition activity of all crude and fractionated extracts were significant at p<0.01.
DPPH free radical scavenging activity
The DPPH scavenging effect of H fomes bark and leaf crude extract was found 56.31% and 52.51%, respectively as compared to reference standard quercetin 66.82% at 50μg/ml concentration (Figure 2a&2b). The IC50 values of bark and leaf extracts was 31.89 µg/ml and 47.32 µg/ml respectively as compared to quercetin with IC50 7.63μg/ml (Figure 2b). The DPPH free radical scavenging effect was substantially increased in the chromatographically fractionated extract (HFBPF1: 80.94% with IC50 15.01μg/ml. HFBPF2: 76.75% with IC50 22.84μg/ml and HFLPF: 80.31% with IC50 24.05μg/ml).
Total flavonoid content
A rapid rise in the increment of flavonoid content in chromatographically fractionated extracts was observed. The increment of flavonoid content was about 28 fold in bark fraction 1 (HFBPF1), 23 fold in bark fraction 2 (HFBPF2) and 46 fold in leaf extract (HFLPF) (Figure 2c).
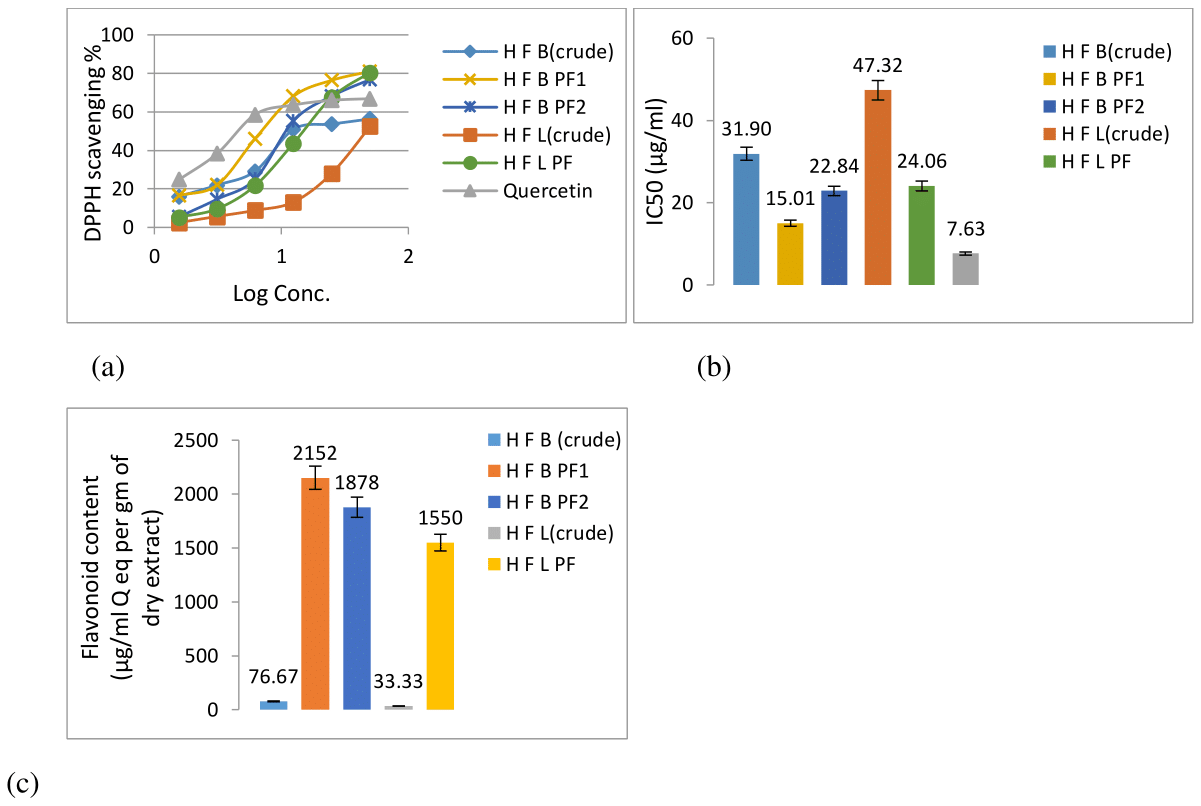
Figure 2 Antioxidant activity and content of H fomes bark and leaf crude and fractionated extracts: (a) DPPH free radical scavenging activity; (b) IC50 values; (c) Total flavonoid content (μg/ml quercetin equivalent/gm of dried extract).
HFB, H fomes bark; H F B PF1, H fomes bark purified fraction 1; H F B PF2, H fomes bark purified fraction 2; H F L, H fomes leaf; H F L PF, H fomes leaf purified fraction
Serine protease inhibition assay
Protection of degradation of casein provided by the different concentration of crude and fractionated plant extract was evaluated. The percent inhibition of trypsin activity and IC50 values are shown in Figure 3a & 3b. Inhibition of trypsin activity was boosted in bark and leaf fraction. The bark fraction1 (HFBPF1) demonstrated the highest trypsin inhibition potential (71.58% with IC50 75.62 μg/ml) as compared with reference stadard quercetin (85.16% with IC50 73.61μg/ml). Concentration dependent inhibition of trypsin activity was observed for all tested extracts (Figure 3a).
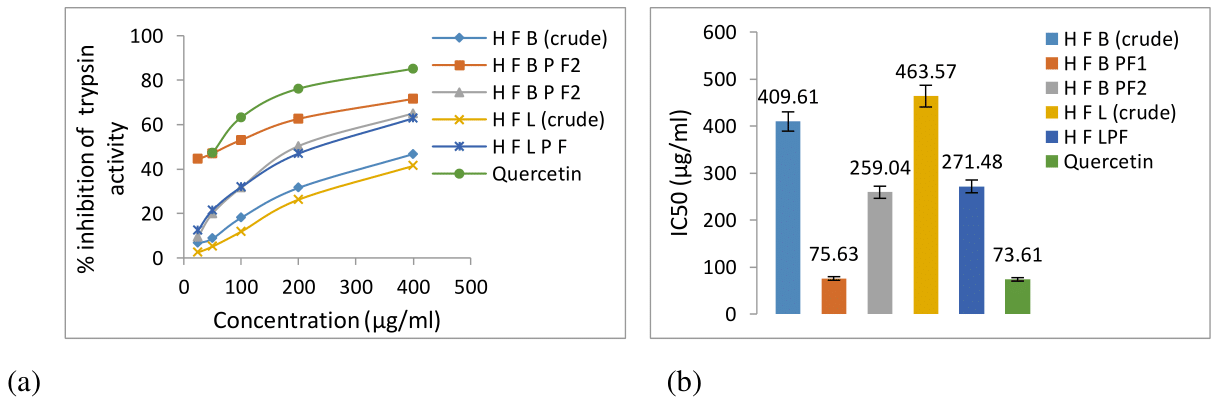
Figure 3 Serine Protease (trypsin) inhibitory activity of ethanolic bark and leaf crude extracts and chromatographically fractionated extracts of H fomes. (a) Percent inhibition of serine protease (trypsin) activity; (b) IC50 (μg/ml) values of tested crude and fractionated extracts.
Two-way ANOVA analysis revealed significant differences between control and treatment of crude and fractionated extract at p˂0.01. Therefore, significant effect in serine protease inhibition was exhibited by H fomes bark and leaf crude extracts as well as their fractionated extracts.
LC-MS/MS analysis
Compound spectrum, MS spectrum, MS/MS spectrum was generated with MS/MS scan using Agilent Technology. Approximately ten compounds were identified by comparing and searching based on the mass, mass/charge ratio and retention time (Table 1 & Figure 4,5).
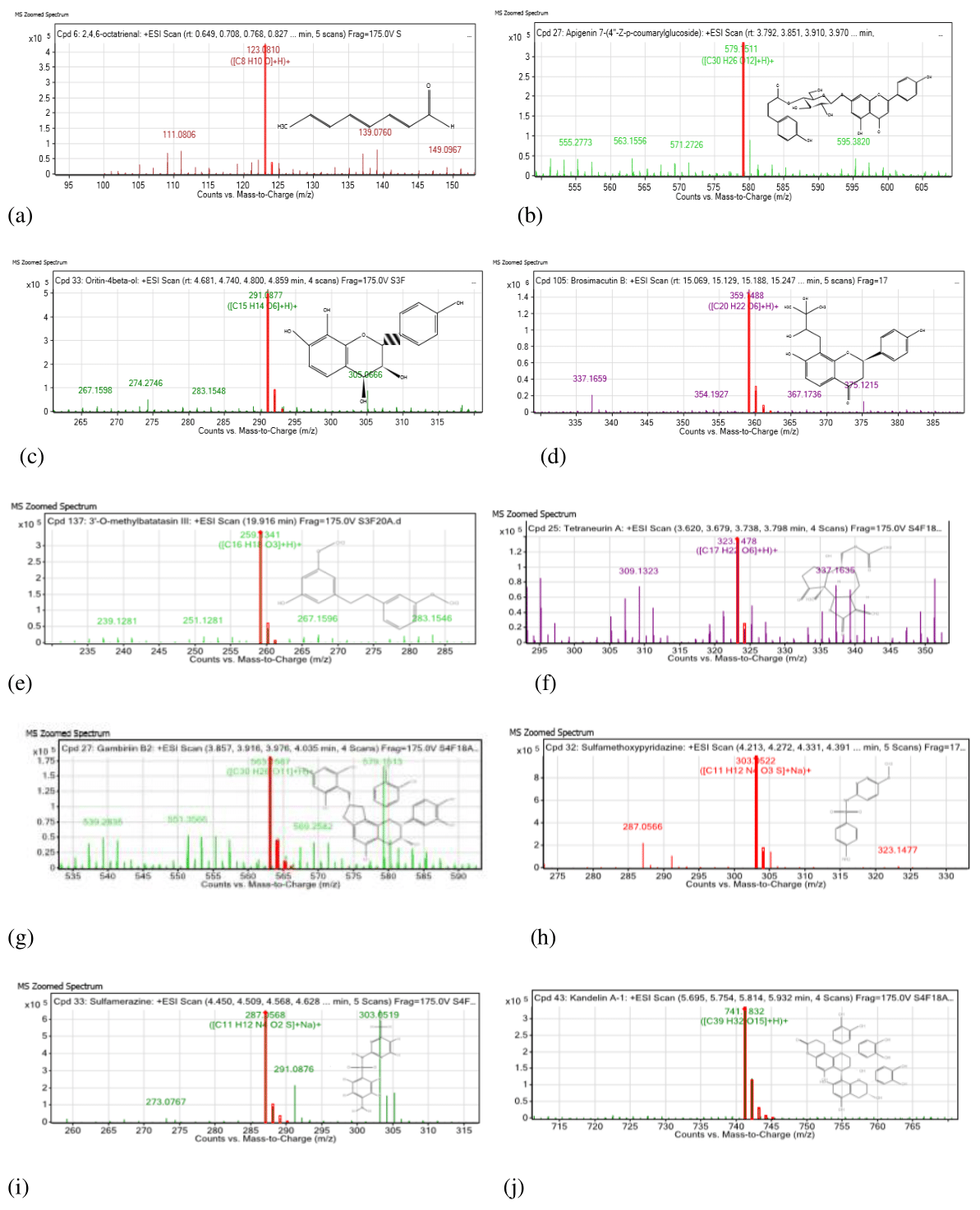
Figure 4 MS zoomed spectrum of the compound identified from chromatographic fractions of ethanolic bark and leaf extract of H fomes. (a) 2,4,6-octatrienal; (b) Apigenin 7-(4˝-Z-p-coumarylglucoside); (c): Oritin- 4 beta-ol; (d) Brosimacutin B; (e) 3̍-O-methylbatatasin; (f) Tetraneurin A; (g) Gambrin B2;
(h) Sulfamethoxypyridazine ; (i) Sulfamerazine; (j) Kendalin A1
|
Sample/ fractions |
SL no. |
Formula |
m/z |
Mass |
Name of the compound |
|
HFBPF1 |
1 |
C8H10O |
123.081 |
122.07 |
2,4,6-octatrienal |
|
2 |
C30H26O6 |
579.1512 |
578.14 |
Apigenin 7-(4˝-Z-p-coumarylglucoside) |
|
|
3 |
C15H26O6 |
291.0875 |
290.0803 |
Oritin- 4 beta-ol |
|
|
4 |
C20H22O6 |
359.1481 |
358.1408 |
Brosimacutin B |
|
|
5 |
C11H2N4O |
207.0306 |
206.0234 |
Not identified |
|
|
HFBPF2 |
1 |
C8H10O |
123.081 |
122.07 |
2,4,6-octatrienal |
|
2 |
C30H26O6 |
579.1512 |
578.14 |
Apigenin 7-(4˝-Z-p-coumarylglucoside) |
|
|
3 |
C15H26O6 |
291.0875 |
290.0803 |
Oritin- 4 beta-ol |
|
|
4 |
C20H22O6 |
359.1481 |
358.1408 |
Brosimacutin B |
|
|
5 |
C8H10O |
259.1341 |
258.1271 |
3̍-O-methylbatatasin |
|
|
6 |
C9H4N |
149.0246 |
126.0354 |
Not identified |
|
|
7 |
C16H10N4O2 |
291.0882 |
290.0809 |
Not identified |
|
|
HFLPF |
1 |
C17H22O6 |
323.1478 |
322.1404 |
Tetraneurin A |
|
2 |
C30H26O11 |
563.1567 |
562.1493 |
Gambrin B2 |
|
|
3 |
C30H26O12 |
579.1517 |
578.14 |
Apigenin 7-( 6˝-p-coumaryldahyde) |
|
|
4 |
C11H22N4O3S |
303.0522 |
280.063 |
Sulfamethoxypyridazine |
|
|
5 |
C11H12N4O2S |
287.0568 |
264.0678 |
Sulfamerazine |
|
|
6 |
C39H32O15 |
741.1832 |
740.1758 |
Kendalin A1 |
|
|
7 |
C20H22O6 |
359.1481 |
358.1408 |
Brosimacutin B |
|
|
|
8 |
C14H226N2 |
223.2186 |
222.2113 |
Not identified |
Table 1 LC-MS/MS compound profile of chromatographic fractions of ethanolic bark and leaf extracts of H. fomes
15-Lox is an enzyme which oxidizes fatty acid and produces proinflammatory mediators.1 This proinflammatory ecocianoids causes release of cytokines resulting inflammation. The elevated level of cytokines triggers activation of blood coagulation pathways. Trypsin and collagenase are some of serine protease enzymes which activate blood coagulation pathway through activation of tissue factor VII. Antioxidant mostly flavonoids possesses a wide spectrum of bioactivity and inhibit a number of enzymes.13 Flavonoid has been shown to inhibit lipoxygenase activity14 as well as serine protease activity.11
In this study, we examined 15-Lox and serine protease inhibitory as well as antioxidant activity of the ethanolic bark and leaf crude extract of H fomes. In the crude extract, we observed an optimistic and significant 15-Lox inhibitory activity with IC50 117.14μg/ml and 137.49μg/ml in bark and leaf extract, respectively. Likewise, bark and leaf crude extract demonstrated significant serine protease inhibitory activity with IC50 409.61 and 463.56 μg/ml. Furthermore, potent antioxidant activity and flavonoid content was observed in these crude extracts.
The substantial increase in 15-Lox and serine protease inhibitory activity as well as antioxidant activity and content was observed in all three chromatographic fractionations (HFBPF1, HFBPF2 and HFLPF (Figure 1 a, b). The fraction with best performance (HFBPF1) exhibited 15-Lox inhibition activity (IC50 62.68 μg/ml as compared to standard quercetin with IC50 54.39 μg/ml), serine protease inhibition activity (IC50 75.63 μg/ml as compared to standard quercetin with IC50 73.61 μg/ml) and DPPH• scavenging activity (IC50 15.01 μg/ml as compared to standard quercetin with IC50 7.63 μg/ml). The other two fractions (HFBPF2 and HFLPF) similarly showed increased potential in the same assays (Figure 1–3). The total flavonoid content also increased in parallel in the active fractions (Figure 2c). About 28 fold increment of flavonoid content was observed in HFBPF1 indicating substantial enrichment of total flavonoid content in the active fractions. These enriched flavonoids could have exerted increased enzyme inhibition as well as antioxidant activity in the chromatographic fractions.
The findings of LC-MS/MS analysis also support the presence of flavonoids and the enrichment of some of the flavonoid compound in the chromatographically separated active fractions (HFBPF1, HFBPF2 and HFLPF). The compound revealed from HFBPF1 and HFBPF2 was similar. It indicates the similar polarity of the two bark fractions. We hereby report the presence of 2,4,6-octatrienal, apigenin, oritin-4-beta-ol, brosimacutin B and 3-0-methyl betatasin in the bark fractions and tetraneurin A, Gambrin B2, apigenin, sulphamethoxypyridazine, sulfemerazine, kandelinA1 and brosimacutin B in the leaf fraction.
Among the identified compound, 2,4,6-octatrienal is an aldehyde having cardioprotective vasodialation activity assessed in rat. It provides cardio protection through upregulation of BCL 2 protein and increased phosphorylation of Akt protein.15 Apigenin is a common dietary flavonoid having reported strong anti-inflammatory and antioxidant activity. Apigenin is being used in cancer therapy which modulates PI3K/Akt, MAPK/ERK, Jak/STAT, NF-κB and wnt/-catenin pathway.16 Tetraneurin is an isoprenoid having antiviral activity against hepatitis C virus.17 Sulfamethoxypyridazine and sulfamerazine are sulphonamide antibacterial compound recommended in vaginal irritation, severe acute thrush, burn injuries and eye infection. These two compounds are available in pharmaceutical formulation. The study on biological function of brosimacutin B, gambrin B2 and kandelin A1 is still required. The antioxidant, anti-inflammatory and anti-cancer activity of the above mentioned compound might be achieved by 15-Lox inhibition activity. The vasodialatory property is contributed by anticoagulation activity through serine protease inhibition activity.18 Thus, the identified compound might contribute to anti-inflammation and anti-coagulation through free radical scavenging, 15-Lox inhibition and serine protease inhibition activity.
β-sitosterol, stigmasterol, stigmast 4-en-3-one are some steroid and epicatechin, procyanidines are the flavonoid reported previously from H. fomes.6,19 Thus, we are reporting the presence of 2,4,6-octatrienal, apigenin, oritin-4-beta-ol, brosimacutin B, 3-0-methyl betatasin, tetraneurin A, Gambrin B2, apigenin, sulphamethoxypyridazine, sulfemerazine and kandelin A1 in H. fomes for the first time.
The increased activity in the chromatographic fractions might be exerted by the enriched antioxidant compounds. Mixture of flavonoid could have synergistic effect in antioxidation through free radical scavenging and enzyme inhibition. Therefore, enriched flavonoid mixture could have better effect and hence it could be a better therapeutic approach as the traditional medicine consists of mixture of herbal constituents.20–22 Recently flavonoid (eg. diosmin, hesperidin, naringin) enriched fraction from citrus fruit has been using in pharmaceutical preparation. They exert a variety of pharmacological properties such as anti-inflammatory, antioxidant and free radical scavenging and antiulcer effects.23
15-Lox is involved in progression of various inflammatory diseases through oxidation of fatty acids and production of inflammatory cytokines thereby. Inflammatory cytokines are the major mediators involved in coagulation activation through activating the tissue factor VII which in turn activated by the cleavage serine proteases. Plant metabolites play role in inti-inflammation and anti-coagulation through antioxidation activity by scavenging of free radical and inhibiting various enzyme activity. H. fomes possesses significant 15-Lox and serine protease inhibition potential which is contributed by the flavonoid compound such as apigenine, brosimacutin, tetranurin A etc present in its bark and leaf extracts. Chromatographic separation successfully enriched the flavonoid content. Thus, the flavonoid enriched fraction of H. fomes bark and leaf could serve in therapeutic application in inflammatory diseases with further clinical investigations.
Experimental facilities were provided by Biochemistry and Molecular Biology Laboratory, Khulna University, Bangladesh. LC-MS/MS facilities was supported by University of Malaya, Malyasia. We acknowledge the Sundarbans East Division, Ministry of Environment, Forest and Climate Change, Government of Bangladesh for necessary administrative and logistic support in collecting plant sample material.
The authors declare that they have no conflict of interest.
The work was financially supported by Grant for Advanced Research in Education (2016-17/64), Ministry of Education, Government of Bangladesh.

©2024 Emdadul, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.