International Journal of
eISSN: 2574-9862


Research Article Volume 9 Issue 2
1Principal Scientist at Regional Station, ICAR-Directorate of Poultry Research, India
1Veterinary Hospital, Dubai Safari Park Section, Dubai Municipality, United Arab Emirates
2Wildlife Pharmaceuticals Pty (Ltd), Mpumalanga, South Africa
3Operations unit, Dubai Safari Park Section, Dubai Municipality, United Arab Emirates
Correspondence: Dr Murad B. M. Mustafa, Veterinary Hospital, Dubai Safari Park Section, Dubai Municipality, United Arab Emirates, Tel 00971506621319
Received: June 17, 2025 | Published: July 10, 2025
Citation: Talukdar A, Swami M. Butorphanol–Azaperone–Medetomidine with ketamine anaesthesia for a dental procedure in a pygmy Hippopotamus at Dubai safari park. Int J Avian & Wildlife Biol. 2025;9(2):58‒61. DOI: 10.15406/ijawb.2025.09.00233
A combination of butorphanol, azaperone, and medetomidine with ketamine was used for the anaesthesia of a pygmy hippopotamus (Choeropsis liberiensis) to facilitate the trimming of an overgrown canine tooth. The animal exhibited adequate sedation and analgesia, enabling the procedure to be carried out safely. Anaesthetic effects were successfully reversed with atipamezole and naltrexone. This case report aims to share practical insights for reference by veterinary professionals.
Keywords: butorphanol, medetomidine, azaperone, pygmy hippopotamus, dental trimming
In the wild, the pygmy hippopotamus (Choeropsis liberiensis) is found primarily in the rainforests of West Africa, with the largest populations in Sierra Leone, Guinea, Côte d’Ivoire, and Liberia.1 The species is listed as Endangered on the IUCN Red List and is included in Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Pygmy hippos are elusive and largely nocturnal, which limits our understanding of their ecology. Unlike the common hippopotamus, they are less gregarious, typically found alone or in pairs. They inhabit swamps, riverbanks, and dense forest, with a strong affinity for water.
In the wild, pygmy hippos face threats such as habitat loss, poaching, and human–wildlife conflict. In captivity, they commonly suffer from poor reproductive success, dental issues, obesity, polycystic kidney disease, stress-related disorders, stillbirths, and elevated early mortality.
There are few published anaesthetic protocols specific to pygmy hippos. However, they present challenges like those seen in common hippos.2 Anaesthetic protocols have improved significantly with the development of newer drug combinations.
Similar to their larger counterparts, there are occasions in which the pygmy hippo may require only sedation or short periods of restraint for tusk work or wound care. Ketamine at 1 mg/kg has been used successfully to immobilize pygmy hippos for short periods.
Bouts et al.,3 report a case series involving 14 pygmy hippos (≈ 250 kg), darted IM with medetomidine (0.08 mg/kg) + ketamine (1.2 mg/kg), followed by isoflurane maintenance. They intubated all animals and used atipamezole (0.4 mg/kg IM) for reversal at the procedure’s end. Monitoring of vital parameters showed stable heart rate, respiratory rate, end-tidal CO₂, and oxygen saturation especially when supplemental O₂ was provided. Recoveries were uneventful in all cases.
Stalder et al.,4 evaluated 10 adult male common hippos undergoing castration with medetomidine (60–80 μg/kg) + ketamine (1 mg/kg). Anaesthesia onset was reliable (~27 ± 12 min), lasting ~97 ± 35 min, with apnoea noted in half of the animals—but without detrimental effects on blood gases.
Miller et al.,2,5 mentioned the combination of Detomidine/Medetomidine with Butorphanol often provides deep sedation for minor procedures, but animals may arouse upon external stimulation.
Pearce reported twenty-one anaesthetic events using different drugs over a 1-year period in a single animal. Etorphine, either alone or combined with acepromazine, together with xylazine induced satisfactory sedation with only minor involuntary leg movements.
The use of detomidine and butorphanol has been reported as a sole anesthetic protocol and for anesthesic induction but only for minor nonsurgical procedures. Spontaneous arousal of animals following stimulation makes this protocol suitable for deep sedation but for non-invasive procedures only. The purpose of the study reported here was to establish a reliable and safe anaesthesia protocol suitable for longer procedures and surgical interventions in hippopotami, with a combination of medetomidine and ketamine. Additionally, we sought to determine species-specific effects of anesthesia in common hippopotami. Intramuscular detomidine or medetomidine mixed with butorphanol has also been described but animals became aroused upon stimulation.2,5
Miller et al.,2 mentioned about use of oral premedication with midazolam; diazepam and detomidine prior to immobilization with alpha 2 agonist butorphanol combination with or without ketamine refereeing to personnel communication with J. Napier, Z Gyimesi and E. Baitman.
Midazolam-zolazepam-tiletamine caused mild sedation. Ketamine-butorphanol has been used for induction of anaesthesia but required supplemental drugs.2,5 A case report mentioned the use of multiple injections of different drugs (atropine, ketamine, butorphanol, detomidine) for induction of anaesthesia in a single pygmy hippopotamus, followed by isoflurane after endotracheal intubation.
Ketamine (1–1.2 mg kg−1) and medetomidine (0.08 mg kg−1) have been used successfully for induction of anaesthesia in pygmy hippopotami. An endotracheal tube was placed, and anaesthesia maintained with isoflurane. Dental procedures, general health examinations and a broncho-alveolar lavage were performed with this.3,6
Miller et al.,2 mentioned their personnel communication to Morris regarding use of Etorphine (2–3 mg) combined with xylazine (100 150 mg) can be used to induce and maintain general anaesthesia in pygmy hippos. The same complications with narcotics are observed in this species as seen with common hippos. Reversal with yohimbine or atipamezole and naltrexone is described above. Reversal agents are used in the same way as those for common hippos with this combination. Another combination that has shown promise is medetomidine-butorphanol. The dosage used was 0.2 mg/kg butorphanol and 0.036 mg/kg medetomidine IM. This was reversed with atipamezole and naltrexone. Similar to the effect in common hippos, both 2-agonist-butorphanol combinations could result in an animal that was able to be aroused with stimulation.
A 6-year and 10-month-old female pygmy hippopotamus (named Ophelia) at Dubai Safari Park was presented with an outward overgrowth of the left lower canine. Initially, the condition did not impact the animal’s welfare. However, by late April 2025, the overgrown tooth began contacting the cheek during mastication, resulting in a visible abrasion. A decision was made to sedate the animal, trim the overgrown canine, and examine the upper left canine, which showed signs of uneven wear.
The dental formula in pygmy hippo varies from common hippo as pygmy hippo has only one pair of incisors on lower jaw compared to two pairs of incisors in common hippo. The dental formula of pygmy hippo is I 2/1, C 1/1, P 3/3, M 3/3 and for common is incisors (I) 2/2, canines (C) 1/1, premolars (P) 3-4/3-4, molars (M) 3/3. The molars are used for mastication of food and the tusk-like canines are used for fighting. The canines are sharpened by constant wear against the shorter upper canines (Figure 1).7
The incisor and canine teeth of hippos are aradicular hypsodont teeth whereas, the molars and pre-molars have bunodont brachydont characteristics.6
Anaesthetising pygmy hippos is challenging due to their unique physiology, which shares similarities with marine mammals. They can enter a "dive reflex" during sedation, leading to centralised blood flow and an increased risk of respiratory depression. Dubai's extreme summer climate further complicates procedures. To mitigate heat stress, sedation was scheduled for early morning, and the animal was kept under intermittent cool water sprinklers to maintain skin moisture and prevent hyperthermia.
The animal was fasted for 24 hours and denied access to the pool for 12 hours prior to sedation. Sprinklers continued throughout the pre- and post-anaesthetic period.
The animal’s last recorded body weight two months prior was 165 kg and has visibly added some body weight in the past two months. The body weight was estimate of 180 kg on the day of the procedure. Dosages were calculated accordingly.
Anaesthetic protocol
The drugs were administered via a 3 ml dart (38 mm Tele-inject needle, Vario K1540V) using a G.UT50 Tele-inject CO₂ system at 06:55 am. The dart was delivered to the left neck, and full delivery of the contents was confirmed (Table 1).
|
Drug |
Dose-rate (mg/kg) |
Total dose (mg) |
|
|
1 |
Medetomidine |
0.04 |
7 |
|
2 |
Butorphanol |
0.11 |
20 |
|
3 |
Azaperone |
0.11 |
20 |
Table 1 Anaesthetic protocol
Anaesthetic timeline
The animal was blindfolded, loaded onto a stretcher, and placed on a stainless-steel worktable. Oxygen supplementation was provided via nasal cannula at 5 L/min, it should be mandatory to supplement oxygen in this species during anaesthesia (Table 2).3
|
|
Time |
Observation/action |
|
1 |
6:55 |
Time of darting the anaesthetic dart. |
|
2 |
6:59 |
Onset of ataxia, head lowering observed. |
|
3 |
7:03 |
Sternal recumbency achieved, Sound stimulus caused arousal, standing resumed. |
|
4 |
7:13 |
Sternal recumbency regained; pinna and skin reflexes intact. |
|
5 |
7:14 |
200 mg ketamine + 1 mg medetomidine hand injected intramuscularly. |
|
6 |
7:20 |
Deep sedation achieved; animal unresponsive to stimuli. Loaded onto a stretcher. |
Table 2 Anaesthetic timeline
A full oral examination was conducted. The left lower canine was trimmed, leaving approximately 4 cm above the gum line. The upper left canine was also trimmed and manually smoothed using a rasp file A dental radiograph confirmed hypsodont canines with elongated crowns and deeply seated roots. As the pulp cavity was not exposed, endodontic (root canal) treatment was deemed unnecessary. A concurrent transrectal ultrasound assessed the ovaries, uterus, and kidneys, all of which appeared normal in shape and size (Figure 2-4, Table 3 ).
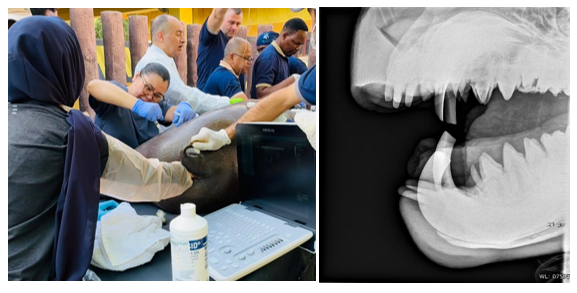
Figure 2 The right lateral projection of the skull was taken using Xprime portable Xray generator, with exposure setting of 1.6mAs and 55KV. And transrectal ultrasound was performed.
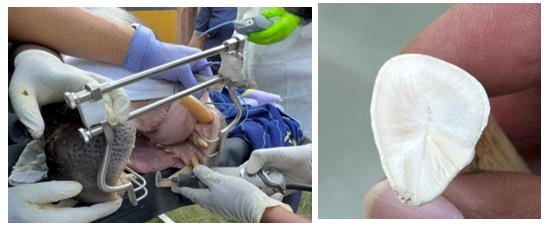
Figure 3 Tooth trimming was performed using handheld mini electric grinder, cut surface of the tooth revealed no exposure of pulp cavity.

Figure 4 An intravenous catheter was placed in the right medial cephalic vein for blood sampling and fluid therapy.
|
|
Time |
Heart rate |
Respiratory rate |
|
1 |
7:13 |
----- |
4 |
|
2 |
7:35 |
----- |
8 |
|
3 |
7:42 |
88 |
----- |
|
4 |
7:50 |
77 |
----- |
|
5 |
7:51 |
81 |
----- |
Table 3
Anaesthetic reversal
After a 60-minutes of first anaesthetic dart, the animal was moved back to its indoor den. Reversal was achieved with:
The animal regained head control and stood within 3 minutes. A smooth recovery was observed, with all reflexes returning within 10 minutes. Post-procedural monitoring continued throughout the day and night. The animal was given access to the pool by the evening. No changes were observed in behaviour, feeding, or social interactions, indicating the absence of pain or post-operative distress (Figure 5).
The anaesthetic protocol used in this case provided smooth induction, sufficient depth of sedation, and an uneventful recovery. Although initial ataxia was achieved at 4 minutes, it took 19 minutes to reach an approachable level of anaesthesia. A supplemental intramuscular dose of medetomidine and ketamine enhanced sedation depth. No adverse reactions or signs of pain were noted throughout the procedure. This protocol may be recommended for minor surgical and dental procedures in pygmy hippopotamus. However, more studies with such anaesthetic protocol need to be carried out for standardizing the combinations.
The authors are grateful to the Dubai Safari Park Management for their support during the procedure. We also convey our gratitude to the Zoology section with special mention of Animal Curators Ms. Anna Mae, Mr. Scott Chambers, Mr. Miguel Santos. Head Ungulate Keeper; Steven Busuulwa, in addition, to Zoo keeping staff. Special thanks to our lab. team and the nursing staff for their valuable contribution.
The authors declare that they have no conflict of interests.

©2025 Talukdar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.