eISSN: 2576-4462


Research Article Volume 9 Issue 2
1PhD in Tropical Agriculture, Researcher at Federal University of Espirito Santo, Alegre - ES, Brazil
2PhD in Cellular and Molecular Biology, Department of Agronomy, Professor at the State University of Ponta Grossa - PR, Brazil
3PhD in Food Sciences, Professor of the Postgraduate Program in Pharmaceutical Sciences and the Postgraduate Program in Food Science and Technology, State University of Ponta Grossa, Ponta Grossa - PR, Brazil
4PhD in Analitical Chemistry, academic department of teaching, Professor at the Federal Technological University of Paraná
5PhD in Phytotechnics, Department of Agronomy, Professor at the University of Espirito Santo, Alegre -ES, Brazil
Correspondence: Ricardo Antonio Ayub, PhD in Cellular and Molecular Biology, Department of Agronomy, Professor at the State University of Ponta Grossa - PR, Brazil
Received: April 11, 2025 | Published: April 30, 2025
Citation: Coutrim RL, Ayub RA, Olivato JB, et al. The use of absorbent sachets in the quality and post-harvest conservation of ‘BRS Fascínio’ peaches. Horticult Int J. 2025;9(2):82-87. DOI: 10.15406/hij.2025.09.00327
Active packaging is an innovative technology that allows direct interaction between the product and the environment, extending its lifespan. This study aimed to evaluate the effect of sachets containing moisture-absorbing substances (zeolite), ethylene (potassium permanganate), antimicrobial (silver nitrate), and oxygen (iron) on the preservation of the post-harvest quality of ‘Fascínio’ peaches stored at refrigerated and ambient temperature. The treatments consisted of the application of 1 gram of the absorbing substances (zeolite, potassium permanganate, silver nitrate, and iron) packed in non-woven fabric (TNT) sachets. These sachets were then inserted into the center of expanded polystyrene packages containing the fruit. Refrigerated storage was carried out at 7 °C with assessments at 0, 9, 18, and 27 days, while evaluations at ambient temperature were carried out at 0, 3, 6, and 9 days. The results indicate that sachets containing potassium permanganate and iron proved to be an efficient strategy for preserving the post-harvest quality of peaches. Both favored the preservation of peel color, flesh firmness and a reduction in changes in soluble solids, total titratable acidity (TTA), hydrogenionic potential (pH), ratio (SS/ATT) and ascorbic acid, extending the shelf life of the fruit for up to nine days at room temperature and 27 days under refrigeration at 7 °C. Therefore, the use of these sachets represents a viable alternative for the transportation and marketing of peaches, contributing to a reduction in post-harvest losses.
Keywords: preservation, active packaging, sachet, prunus persica
The peach (Prunus persica (L.) Batsch) is a temperate-climate fruit belonging to the Rosaceae family. Recognized worldwide for its organoleptic characteristics, such as juiciness, aroma, sweetness, and nutritional value,1 its global production in 2022 was 26.3 million tons (FAO, 2022). In Brazil, production reached 208,823 thousand tons.2 However, post-harvest losses account for 40 to 50% of total production, which limits its distribution over long distances, reduces availability, and negatively impacts market value.3 These losses are attributed to physical, pathological, and physiological deterioration.4
To mitigate these losses, post-harvest techniques have been implemented under promising results. Among these techniques, non-intrusive methods that do not come into direct contact with the fruit5 are prominent. A promising example of these methods is the use of absorbent sachets. These sachets remove undesirable compounds and processes that interact with the food, such as oxygen, excessive water, ethylene production, and microorganisms inside the packaging, which extends the shelf life of the fruits and preserves their quality and safety during storage.6 The main absorbing substances widely studied include potassium permanganate, used as an ethylene absorber; silver nitrate, with antimicrobial action; zeolite, used as a moisture absorber; and iron, effective as an oxygen absorber.
Potassium permanganate (KMnO4) is a strong oxidizing agent that promotes the rapid conversion of ethylene into carbon dioxide, manganese dioxide, and potassium hydroxide. During the reaction, the sachets containing KMnO4 change color from violet to brown, which indicates the saturation and elimination of ethylene. This substance, which acts in the oxidation of ethylene, can be applied at different stages of the production chain, such as storage, transportation, and commercialization.7
Silver nitrate (AgNO3), in turn, releases silver ions (Ag+), that replace the hydrogen cations (H+) present in the sulfhydryl or thiol groups (-SH) of sulfur-containing proteins through electrostatic attraction or affinity.8 Once absorbed by cells, silver ions inactivate respiratory enzymes, generate reactive oxygen species, and cause the rupture of cell membranes, resulting in the death of microorganisms.9
Zeolite a microporous crystalline aluminosilicate composed of tetrahedral units of AlO4 and SiO4 plays the role of a hygroscopic material. It absorbs moisture from the air in indoor environments through physical adsorption or chemical reaction, until it reaches equilibrium with the environment.10 This mechanism helps minimize food weight loss caused by the released water activity. The iron compound, in turn, reacts with water vapor, forming ferric hydroxide and creating an atmosphere with low residual oxygen levels inside the packaging. This reduction in available oxygen contributes to the control of fruit respiration, which extends its shelf life.11
Based on this context, this study aimed to evaluate the effectiveness of absorbent sachets containing KMnO4, Fe3+, AgNO3, and zeolite, inserted in packaging, in preserving the post-harvest quality of ‘BRS Fascínio’ peaches. The tests were carried out under storage conditions of 7 °C and 21 °C, and the results were compared with the control.
The experiment was conducted at the Laboratory of Fruit Growing Applied to Biotechnology (LabFruti) at the State University of Ponta Grossa (UEPG). It consisted of checking the effects of packaging on the preservation of peaches by analyzing their post-harvest quality. The ‘BRS Fascínio’ peaches were harvested at full maturity and selected based on size and the absence of injuries or signs of pests and/or diseases. After standardization, the fruit was placed in expanded polystyrene trays accompanied by specific sachets.
The sachets were made from a polypropylene material known as “NWF,” sealed with hot glue in a rectangular shape containing the treatments, inserted in the center of the expanded polystyrene trays between the four peach fruits (without contact), and covered with transparent polyvinyl chloride (PVC). The treatments tested included: 1 g of zeolite (moisture absorber), silver nitrate (antimicrobial), potassium permanganate (ethylene absorber), iron (oxygen absorber), and the control (without the sachet).
The experiment followed a completely randomized design (CRD), consisting of five active substances (treatments), with each treatment having four replicates. Each replicate consisted of four fruits with an average weight of 150 grams each. The sets (samples + packaging) were stored in two thermal conditions: refrigerated (7 °C) and room temperature (21 °C). In refrigerated conditions, the evaluations were performed at 0, 9, 18 and 27 days; while in room conditions, the fruits were evaluated at 0, 3, 6 and 9 days.
Post-harvest quality analysis
Colorimetric analysis: Conducted in the equatorial areas of the fruit epidermis with the aid of a Konica Minolta colorimeter, model Chroma Meter CR-400/410. The coloration was determined in the CIELAB color space, with the following parameters: L* (luminosity), a* (green/red coordinate: where negative values indicate green hues and positive values indicate red hues), and b* (blue/yellow coordinate where negative values indicate blue hues and positive values indicate yellow hues). Based on these parameters, the hue angle, which is considered the most accurate indicator of fruit color, was calculated.
The hue angle ranges from 0 to 360°, where 0° (or 360°) corresponds to maximum red, 90° to maximum yellow, 180° to maximum green, and 240° to maximum blue. As the peach’s epidermis transitions from green to yellow, orange or red, the hue angle decreases. In this research, the Angle was calculated using the following equations: h°= tan-¹((b*)/(a*)) when a*>0 and b*>0 and h°= 180+tan-¹((b*)/(a*)) when a*<0 or b*>0.12 Each reading was performed at two different points per fruit.
Firmness: Determined using a manual penetrometer from the Fruit Pressure Tester brand—model FT 327 equipped with an 8 mm diameter tip—where two readings were taken in the equatorial region of the fruit with epiderms. The values were expressed in Newton (N).
Soluble solids (SS): Measured with two drops of juice using a DIGIT handheld refractometer and results expressed in °Brix.13
pH: 5 g of the fruit pulp were weighed and blended in a Mondial blender with 45 mL of distilled water. For measurement, a Hanna pH 21 pH/mV meter (Sigma-Aldrich®) was used. The real values were expressed in pH.
Titratable acidity (TA): Determined by neutralization titration based on the methodology of Carvalho et al.14 The results were expressed as a percentage (%) of citric acid.
Ratio: The SS/AT ratio obtained by dividing the soluble solids and titratable acidity contents.
Ascorbic acid (AA): determined by neutralization titration, based on the reduction of the indicator 2.6-dichloro-phenol-indophenol-sodium (DCFI 0.02%) by ascorbic acid based on the methodology of Strohecker and Henning,15 and expressed in mg 100 g-1 of pulp.
Statistical analysis
The experimental data collected from post-harvest quality analyses were subjected to statistical tests using the Excel 3.0 package (Microsoft corporation, 2025). The data were also subjected to the mean and standard deviation test. The OriginPro® statistical package, version 9.5.1,16 was used to make the graphs. For the two-way interactions for colorimetric analysis using response surface methodology, Minitab® 18 was used.17
Figure 1 shows a two-dimensional representation of the response surface, considering the treatments applied to the fruits (zeolite, silver nitrate, potassium permanganate, and iron) and the storage periods under refrigerated (0, 9, 18, and 27 days) and ambient (0, 3, 6, and 9 days) conditions. The main focus is on the hue angle, a parameter that reflects the color change throughout the ripening of the fruits. At the beginning of storage (Figure 1B), the darker regions indicate angles between 116º and 118º, which correspond to the full maturation stage. At this stage, the fruits exhibit a yellow-green hue attributed to the concentration of carotenoids and the reduction in the green coloration of the epidermis, which results from chlorophyllase synthesis.18
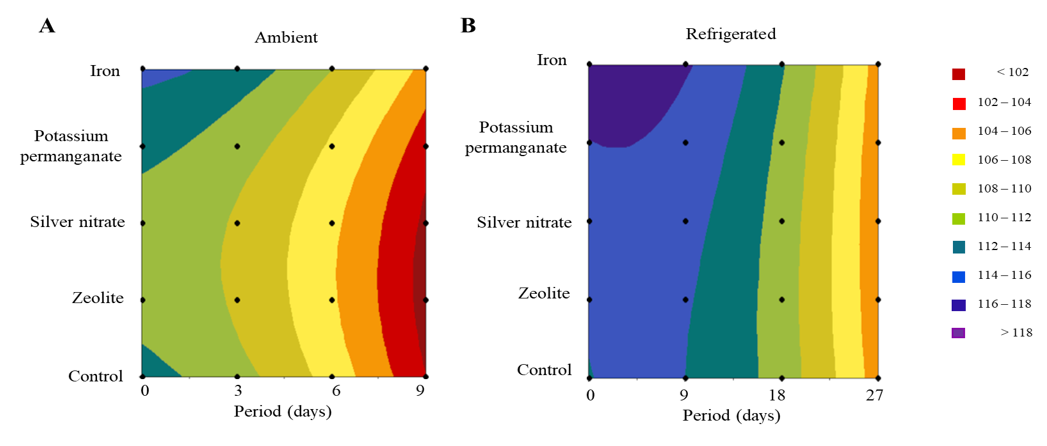
Figure 1 Response surface based on sachets of eolite, silver nitrate, potassium permanganate, iron, and control on the color of ‘BRS Fascínio’ peaches fruits under ambient (A) and refrigerated (B) conditions. UEPG, Ponta Grossa, 2024.
As ripening progresses, a reduction in hue angle values. Under ambient conditions, a gradual change to intense red hues occurs on the ninth day, while orange hues predominate on the 27th day under refrigerated storage. These results demonstrate that temperature directly influences the color change process and accelerates fruit ripening. According to Rinaldi et al.,19 lower temperatures reduce metabolic activity, decreasing the action of enzymes responsible for pigment synthesis. Consequently, fruits stored under refrigeration exhibit a less intense background color.
Regarding the effects of the sachets of zeolite, potassium permanganate, silver nitrate, and iron, compared to the control, there was a significant impact on the quality of the fruit kept at ambient temperature (Figure 1A). The results indicated that the treatments with potassium permanganate (KMnO4) and iron (Fe3+) presented the highest, with spectral values ranging from 106 to 102, characterized by shades ranging from orange to red.
KMnO4 stood out as an effective solution for reducing the concentration of ethylene, a phytohormone associated with fruit maturation. This mechanism contributes to reducing the respiratory rate and metabolic processes, which slows down deterioration. Similarly, the use of sachets containing iron-based powder has proven advantageous due to its limitation of oxygen permeability in packaging. This reduction in oxygen flow directly interferes with respiration rates, color changes, and enzymatic metabolic activities, which promotes the preservation of the quality of packaged and/or stored food products.20
On the other hand, the treatments with zeolite, silver nitrate, and the control presented the lowest averages: spectra below 102, marked by a more intense red color. The low efficiency of natural zeolite can be explained by the limited amount of material in the sachets, which reduced its adsorption capacity and caused rapid saturation. The results of Brujin et al.21 corroborate this observation, as they show that natural zeolite contributed to a marked development of the red color in tomatoes in the first eight days of storage. However, the modification of zeolite through the incorporation of copper and zinc cations proved to be an efficient strategy to contain the color evolution, as it enhances its practical application.
Regarding the silver nitrate (AgNO3), its effectiveness in preserving fruit color was compromised, possibly due to the toxicity generated by the application of 1 g of the substance. According to Technical Regulation No. 326/2019,22 the most efficient way to apply silver nitrate is on the nanometric scale, with particles between 1 and 100 nm. Nanotechnology significantly increases the surface area, as it favors the release of silver ions and intensifies its antimicrobial action.9 This approach enhances the functionality of silver nitrate in applications related to food preservation.
The firmness of a fruit is directly related to the hardness of its flesh, which is influenced by the solubility of the pectic substances present in the cell walls. During ripening, these substances are solubilized and result in the softening of the flesh.23 As shown in Figure 2, the highest firmness averages were observed at the beginning of the evaluations and gradually decreased over the storage period. This decline reflects the natural softening caused by fruit senescence.
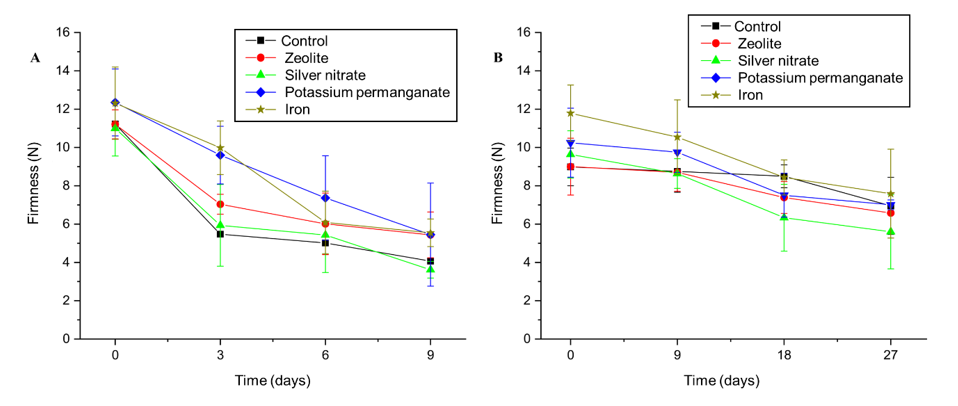
Figure 2 Effect of sachets of zeolite, silver nitrate, potassium permanganate, iron, and control on the firmness (N) of “BRS Fascínio” peach fruits at ambient temperature (A) and refrigerated temperature (B). UEPG, Ponta Grossa, 2024.
On the last day of storage, the fruit treated with zeolite, KMnO4, and Fe3+, which remained relatively firm until the ninth day, showed the lowest firmness losses when kept at ambient temperature (Figure 2A). Under refrigerated conditions, the sachets containing iron (Fe3+) were the most effective. Their lowest firmness losses occurred up to the 27th day, which confirmed their performance in preserving fruit texture (Figure 2B). In contrast, the AgNO3 treatment resulted in greater losses of firmness in both storage conditions, which indicated a lower efficiency in preserving the structural integrity of the fruit.
Storage of peach fruits under refrigerated conditions (7°C) promoted the development of mealiness symptoms, a physiological disorder that negatively affects the sensory quality of the fruits (Figure 3). Mealiness is associated with an imbalance in the activity of pectolytic enzymes during the ripening process. This imbalance is characterized by an increase in the activity of the enzyme pectin methylesterase, which reduces or inhibits the action of polygalacturonase, responsible for the depolymerization of pectin. As a result, pectin undergoes incomplete degradation, which results in the accumulation of free water in the intercellular spaces. This phenomenon forms a gel structure that compromises the texture of the fruit and causes a dry, mealy flesh. In addition, mealiness compromises the juiciness of peaches and alters their sensory perception, providing an unpleasant flavor to consumers.24
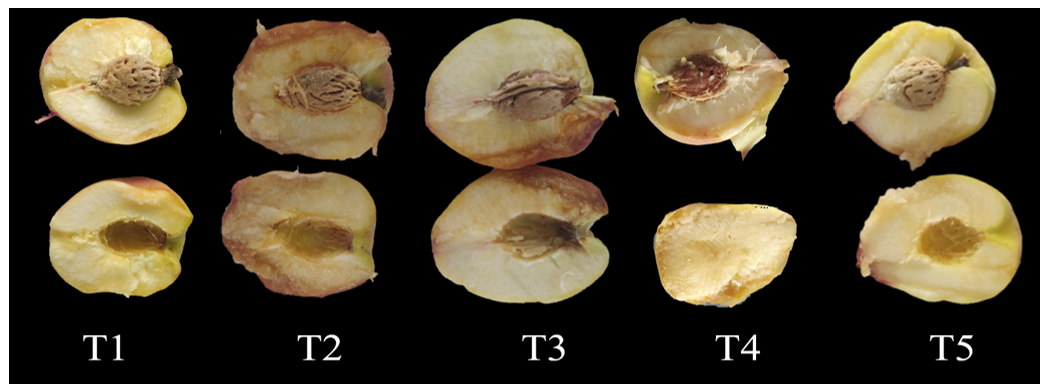
Figure 3 Mealiness symptoms on 'BRS Fascínio' peaches refrigerated at 7 °C after 27 days of storage.
The content of soluble solids content (SS) and total titratable acidity (ATT) are important indicators for assessing fruit maturity and sensory profile. During storage, a decrease in ATT values was observed, due to the metabolic transformations associated with ripening and subsequent deterioration of the fruit (Figures 4A and 4B).
The iron treatment slowed down the reduction in ATT, maintaining higher levels until the ninth day, when the values converged between the treatments in ambient storage (Figure 4A). In the refrigerated condition, the treatment with silver nitrate initially showed higher ATT values. However, on the ninth day, this treatment suffered a reduction in the concentration of organic acids. In contrast, the iron treatment maintained the highest levels of ATT, and in subsequent evaluations, the values between the treatments were equal.
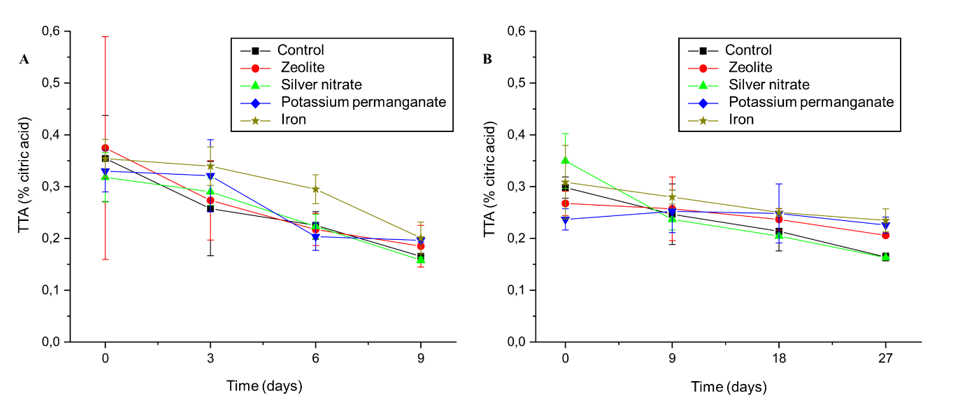
Figure 4 Effect of sachets of zeolite, silver Nitrate, Potassium Permanganate, Iron, and control on the titratable acidity (g of citric acid in 100 mg-¹ of pulp) of ‘BRS Fascínio’ peach fruits at ambient temperature (A) and refrigerated temperature (B). UEPG, Ponta Grossa, 2024.
Characterizing the greater preservation of ATT levels in peach fruit, due to this micronutrient Fe+3 integrating the mitochondrial respiratory chain and acting as a cofactor for antioxidant enzymes, contributing to the reduction of oxidative stress and maintenance of cellular integrity, aspects linked to the degradation of organic acids, prolonging conservation.25
Despite the efficiency of the treatment, given that the commercial shelf life of peaches is short, ranging from 3 to 5 days at room temperature (20 to 25°C) and 10 to 14 days in the refrigerator (0 - 2°C), the reduction observed in the treatments, in general, can be explained by the climacteric characteristic of the fruit, intensification of the metabolic rate, decreasing the efficiency of the substances and accelerating the natural process of fruit senescence.26
The data obtained (Figure 5A) show that, under ambient conditions, treatment with AgNO3 resulted in the greatest increase in TSS values, reaching approximately 13 °Brix on the ninth day. This result suggests that AgNO3 accelerates the ripening process by enhancing the conversion of polysaccharides into simple sugars. Treatment with zeolite showed an increase in TSS, although less marked, reaching 12 °Brix in the same period. In contrast, KMnO4 and Fe3+ maintained more stable values, around 11 °Brix, indicating less influence on ripening.
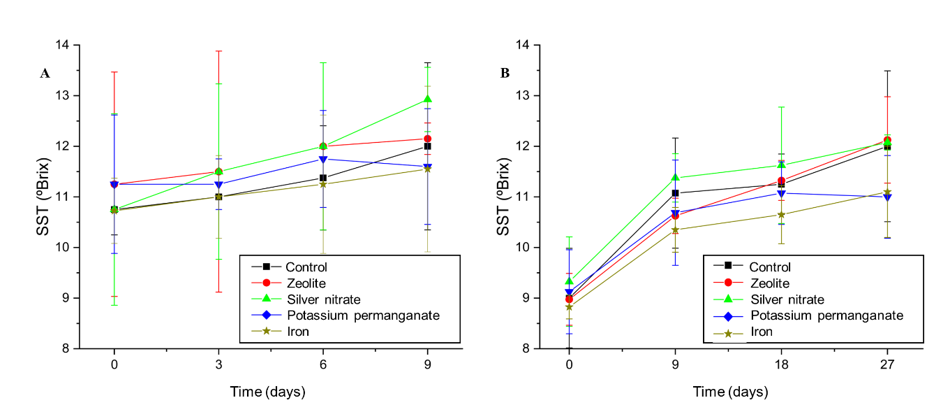
Figure 5 Effect of sachets of Zeolite, Silver Nitrate, Potassium Permanganate, Iron, and control on the soluble solids (°Brix) of ‘BRS Fascínio’ peach fruits at ambient temperature (A) and refrigerated temperature (B). UEPG, Ponta Grossa, 2024.
Under refrigerated storage (Figure 5B), the fruits initially presented similar TSS values (9-10 °Brix), which indicates uniformity in the pre-treatment maturation stage. After nine days, the values converged to approximately 11 °Brix in all treatments, suggesting that refrigeration delayed ripening. However, differences began to emerge on the 18th day, when the control, zeolite, and silver nitrate reached values above 12 °Brix. They accelerate maturation and sugar concentration, but can reduce fruit durability due to the intensification of metabolic processes at temperatures above the ideal (0.5-1.0 °C). In contrast, KMnO4 and Fe3+ maintained lower values, close to 10.5-11.5 °Brix. They proved to be effective in delaying ripening and preserving sugars, and are recommended to extend the shelf life of fruits during refrigeration.
The ratio (TSS/TA) is an index that expresses the balance between total soluble solids (TSS) and titratable acidity (TA). This parameter is widely used as an indicator of the sensory quality of fruit, as it reflects the interaction between sweetness and acidity, characteristics that determine the flavour perceived by the consumer. The quantification and evolution of this index during storage are essential for understanding the ripening and conservation processes, as they guide strategies for preserving post-harvest quality.27
The data presented in Figure 6A shows the evolution of the TSS/TA ratio for the treatments under ambient conditions. The control showed a gradual increase in the index, starting at approximately 40 on the first day and reaching approximately 65 on the ninth day. This behaviour reflects the natural ripening of the fruit, characterized by an increase in sweetness due to the conversion of polysaccharides into simple sugars, accompanied by a reduction in acidity. The treatment with zeolite showed a similar evolution to the control, but with a less pronounced increase in the TSS/TA ratio. This suggests that the zeolite slightly delayed ripening, as it maintained a more stable balance between sweetness and acidity throughout the period.
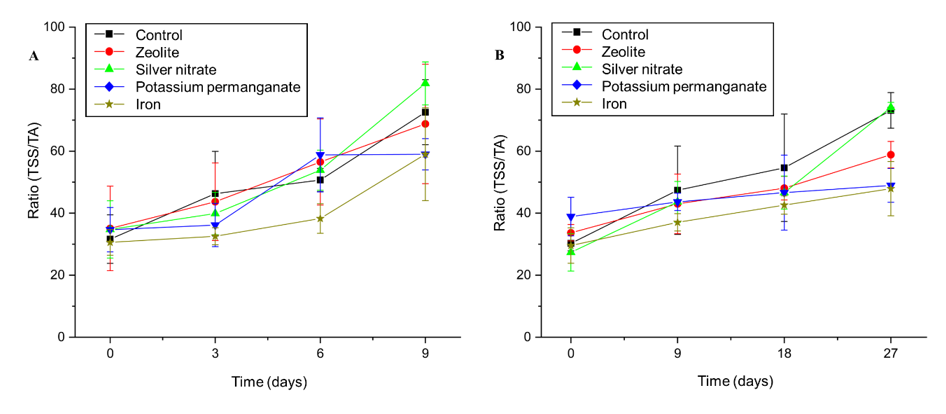
Figure 6 Effect of sachets of Zeolite, Silver Nitrate, Potassium Permanganate, Iron, and control on the relationship between the soluble solids content and the titratable acidity (ratio) of ‘BRS Fascínio’ peach fruits at ambient temperature (A) and refrigerated temperature (B). UEPG, Ponta Grossa, 2024.
AgNO3 promoted the greatest increase in the ratio, reaching values close to 85 at the end of the nine days. This indicates that the substance accelerated fruit ripening, intensifying the relative increase in sweetness in relation to acidity. In contrast, KMnO4 and Fe3+ resulted in the smallest increases in the TSS/TA ratio, ending at around 60. This behavior suggests that these treatments delayed ripening, maintaining a more stable balance between sweetness and acidity.
In the refrigerated storage (Figure 6B), all treatments began with similar TSS/TA values, around 35. The control treatment showed a gradual increase, reaching around 55 at the end of the period (27 days), which shows natural ripening even under refrigeration. Zeolite showed similar behavior to the control, but with a more moderate increase, which suggests a delaying effect on ripening and a balance between sweetness and acidity. AgNO3 was again the treatment that showed the greatest increase in the TSS/TA ratio, reaching values close to 80 at the end of the period. This reinforces its action as a ripening accelerator, intensifying sweetness in relation to acidity. On the other hand, KMnO4 and Fe3+ showed the smallest increases in the ratio, remaining close to 40 in the final assessment. These treatments showed effectiveness in delaying ripening and preserving fruit quality throughout storage.
Vitamin C is a sensitive compound that undergoes degradation during storage, which directly affects the nutritional value of the fruits. The analysis of the behavior of the different treatments under temperature and refrigerated conditions revealed distinct preservation patterns. Under ambient conditions, the control treatment showed an expected decrease in vitamin C levels, from approximately 2.8% on day zero to 2.0% on day nine, reflecting the natural degradation of ascorbic acid during storage (Figure 7A). The zeolite treatment exhibited similar behavior, but with a further reduction, reaching 1.9% at the end of the period, indicating that the zeolite was not effective in preserving vitamin C.
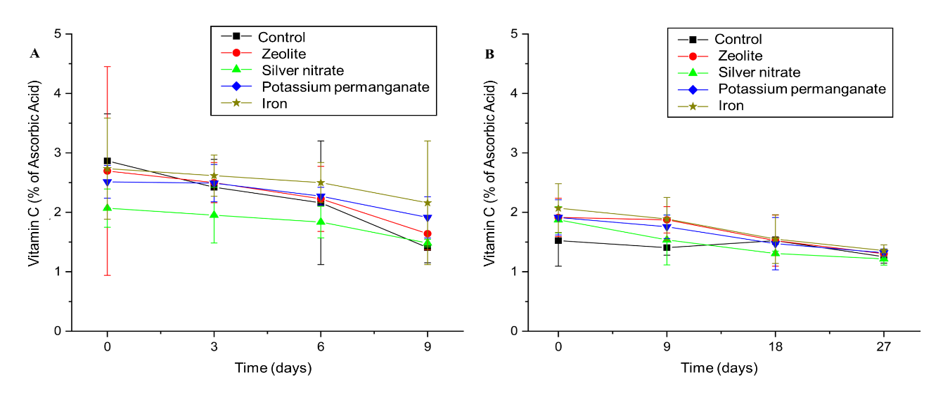
Figure 7 Effect of sachets of Zeolite, Silver Nitrate, Potassium Permanganate, Iron, and control on the vitamin C concentration in ‘BRS Fascínio’ peach fruits at ambient temperature (A) and refrigerated temperature (B). UEPG, Ponta Grossa, 2024.
The silver nitrate treatment exhibited the greatest loss, with its initial content of 2.1% decreasing to 1.5%. This suggests that this treatment might have accelerated the degradation of the ascorbic acid, possibly due to chemical reactions or the stimulation of oxidative processes. In contrast, the potassium permanganate treatment showed greater stability. It kept the vitamin C content close to that of the control (2.0%) at the end of the period, suggesting a moderate preservation effect, although unable to prevent degradation. Iron treatment was superior in preserving vitamin C, reducing its loss from 3.0% to only 2.5%. This highlights iron's role as an effective antioxidant, protecting the fruit from oxidative damage and preserving their nutritional value.
Ascorbic acid degradation was slower under refrigeration (Figure 7B). The control showed a decrease in vitamin C levels, from 1.8% on day zero to approximately 1.5% on day 27, which confirmed that refrigeration slows down but does not eliminate degradation. Zeolite showed a less pronounced reduction, maintaining the content at around 1.7% at the end of 27 days, while iron exhibited a similar behavior, also showing a slight reduction from 2.0% to 1.7%. Both treatments showed a consistent stabilizing effect, significantly slowing vitamin C degradation during refrigerated storage.
At the start of the experiment, the pH of the fruit was similar in all treatments, with values close to 4.0. This result indicates that the fruits were in homogeneous initial conditions, with no significant differences in acidity. After three days of storage, the differences between treatments began to emerge. The iron treatment showed the smallest increase in pH, remaining around 4.2, which indicated more efficient preservation of acidity (Figure 8A). In contrast, the control, zeolite, potassium permanganate, and silver nitrate showed more significant increases, with values ranging from 4.3 to 4.4. This moderate increase in pH reflects the natural degradation of organic acids in the fruits, a characteristic process during ripening.
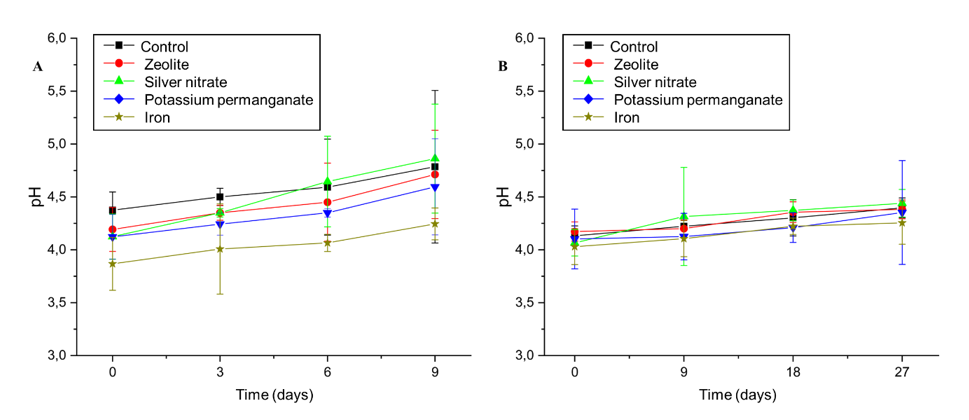
Figure 8 Effect of Zeolite, Silver Nitrate, Potassium Permanganate, Iron, and control sachets on the pH of 'BRS Fascínio' peach fruit at ambient temperature (A) and refrigerated temperature (B). UEPG, Ponta Grossa, 2024.
On the sixth day, the differences between the treatments became more evident. Silver nitrate showed the greatest increase in pH, reaching approximately 4.8, indicating a marked reduction in acidity and a faster progression in the ripening processes. The control, zeolite, and potassium permanganate showed intermediate increases in pH, with values around 4.5, suggesting moderate organic acid degradation. In contrast, iron maintained the smallest increase, with values close to 4.3, evidencing its superiority in preserving acidity for a longer period.
At the end of storage, a consistent increase in pH was observed for all treatments, albeit with significant variations. Silver nitrate reached the highest pH, close to 5.3, indicating a greater reduction in acidity and suggesting that this treatment accelerated fruit ripening. The control, zeolite, and potassium permanganate presented intermediate values that varied between 4.7 and 4.9, reflecting a moderate impact on the acid degradation and ripening processes. Iron continued to demonstrate the smallest increase in pH, remaining around 4.3 until the end of the period, which confirms its effectiveness in preserving acidity throughout storage.
Under refrigerated conditions, the pH behavior also signaled chemical and microbiological natural changes in the fruits over time. The control, initially with a pH close to 4.0, showed a gradual increase, reaching approximately 4.5 at the end of the 27-day period (Figure 8B). The zeolite followed a similar trend to the control, but with slightly higher values at the end, suggesting a slight impact on pH elevation. Silver nitrate was the treatment that altered pH the most, with a significant increase over time, reaching values close to 5.0 on the 27th day. This behavior shows that silver nitrate promotes more pronounced chemical changes, possibly related to accelerated maturation.
Potassium permanganate, on the other hand, showed a more stable behavior, with less variation in pH during the storage period, although the wider error bars indicate greater variability in the data for this treatment. Iron stood out again for presenting the lowest pH values among the treatments, consistently remaining below 4.5. This result suggests that iron interferes minimally in the chemical changes of the fruit, which contributes to a more efficient preservation of acidity.
AgNO₃ and zeolite treatments are more suitable for applications where accelerated maturation is desired, such as immediate consumption. On the other hand, KMnO₄ and iron have potential for post-harvest conservation under refrigerated conditions, ensuring that fruit quality is preserved for longer periods. Future studies should evaluate the effects of these treatments under different storage conditions and fruit varieties.
The authors are very grateful to the students working for the Laboratory of Applied Biotechnology in Fruit Growing for their fundamental assistance along with carefully performed crop analyses belonging to the State University of Ponta Grossa for the support provided. Special thanks are also dedicated to the Federal University of Espírito Santos for being the base of my studies and CAPES (Coordination for the Improvement of Higher Education Personnel) for granting the scholarship.
The author would like to thank the Academic Writing Center of the State University of Ponta Grossa for assistance with English language translation and developmental editing.
The authors Ricardo Ayub, Moises Zucoloto and Juliana Bonametti reviewed all the written work, contributing to a clearer version of the text. Ranyelly Leão was responsible for the execution and complete elaboration of the results and discussions.

©2025 Coutrim, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.