eISSN: 2576-4462


Research Article Volume 9 Issue 2
Melbourne Polytechnic, Department of Arts, Education & AgriTech, Epping VIC 3076, Melbourne Australia
Correspondence: Sadiqul Awal, Melbourne Polytechnic, Department of Arts, Education & AgriTech, Epping VIC 3076, Melbourne, Australia, Tel +61392691162
Received: March 02, 2025 | Published: April 2, 2025
Citation: Taylor R, Awal S. The impact of cigarette butts on soil properties, seed germination and seedlings. Horticult Int J. 2025;9(2):50-61. DOI: 10.15406/hij.2025.09.00323
The effects of cigarette butts (CBs) on soil properties, seed germination, and seedling growth were examined. Plant species used in the experiment were peas (Pisum sativum) and barley (Hordeum vulgare). An indoor environment was used for this potted experiment. The results of pre- and post-experiment soil tests and the control and treatment groups were significantly different. Experiments were documented digitally and mechanically in order to highlight seed germination, vegetative growth, and root health. The seeds of P. sativum from both the control (not exposed to cigarette butts) and the treatment (exposed to cigarette butts) germinated within one week, with the treatments displaying a higher germination and growth rate. Seeds of H. vulgare treated, and control showed similar growth patterns, with the treatments showing higher germination rates. Fresh weights of P. sativum and H. vulgare were significantly heavier than dried weights, indicating higher water content. To provide a baseline for future research, it is imperative to investigate how cigarettes affect soil properties, seed germination, and seedlings in view of the large amount of cigarette butts the environment collects every year.
Keywords: cigarettes butts, soil properties, germination, seedlings, peas, barley
Throughout the past decade, cigarette filters have become the most abundant litter item on the planet. They litter beaches, streets, sidewalks, waterways, and public spaces.1 As the global population grows, the number of cigarettes smoked worldwide is still increasing, even though smoking prevalence may be declining in some regions.2 Thus, even with a lower percentage of smokers, the total number of smokers is increasing due to a greater number of people on the planet; this is a significant public health concern.3 It is estimated that 4.5 trillion cigarette filters are disposed of into the environment every year.4 Globally, there are over six trillion conventional fuel cigarettes produced and consumed every year.4,5 It is estimated that between 24 and 32 billion cigarette butts (CBs) are thrown away in Australia6 of which 10% end up in water ecosystems. According to other studies, the global number of cigarette butts consumed exceeds 5.6 trillion,7-9 which represents an annual global mass of 845,000 tons.10
Approximately 76%-84% of smokers litter their cigarettes filters rather than disposing of them in a bin.11,12 The majority of CBs are discarded on pavements and in green spaces around the world. Since 2014, there have been at least 22 butts found per 1000 m2 in Australia, including in Sydney and Melbourne. The popular St Kilda beach in Melbourne recovered over 4000 CBs within a 25-square-meter area. Each day, Sydney Street cleaners collected 15000 CBs. According to Wallbank et al.,13 global mass clean-ups led to increased funding for street cleaners and cleaning supplies.
A systematic review on the environmental fate of cigarette butts and their toxic effects on aquatic organisms published in 2021 highlights a lack of data on the toxic effects of CBs on aquatic organisms. There is a possibility that increased levels of CB leaching will cause toxicity levels to increase, resulting in fatalities. There is no doubt that smoked CBs have a greater effect on international species of fish, such as Clarias garienpinus, Atherinops affinis, Pimephales promelas, Tidepool snails, and Hymenochirus curtipes, than unsmoked CBs, even though they are more harmful.14 Although in a study Gill et al.,15 claimed that CBs may have low toxicity to soil-dwelling invertebrates.
There have been several studies showing that cigarettes' ingredients can cause serious harm to humans.16-19 The thermoplastic cellulose acetate is commonly used in films, clothing fibres, and a variety of plastic applications. thermoplastic cellulose acetate decomposes into microplastics when exposed to water or the natural environment.20-23 Almost all cigarette butts contain cellulose acetate fibers, a type of bioplastic. It has been reported that both biodegradable and nonbiodegradable CB litters contain a number of toxic metals and metalloids, including aluminium (Al), arsenic (As), boron (B), barium (Ba), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), mercury (Hg), manganese (Mn), nickel (Ni), lead (Pb), antimony (Sb) ), selenium (Se), tin (Sn), strontium (Sr), titanium (Ti), vanadium (V), zinc (Zn).6 As described by Koroleva et al.,6 soil tests showed pH fluctuations, suggesting potential environmental impacts.
The effects of microplastics on the environment and human health are, however, less well understood. Studies are being conducted on the long-term effects of decomposing cigarettes.6 There are various types of soils that decompose tobacco casings and paper casings, including gravel, sand, loam, and clay. Eventually, these chemicals and casings will cover waterways with petroleum.24 As a result, floral, faunal, microeconomic, and bacterial communities suffer from toxic environments, which ultimately adversely affect human drinking water.6 In another study,25 alkaloidal donors are able to absorb alkaloids such as nicotine from plants that accept them. The allelopathic inhibition and hormesis caused by nicotine also cause stress in seeds and seedlings.26 Furthermore, microplastic chemicals are toxic to ecosystems, serve as vehicles for transport, and are added to cigarette butt production to enhance polymer properties and extend microplastic life.27
Despite the fact that long-term cigarette effects on soil are understudied, they have been proven to have significant effects on human health,5 both short-term positive effects and significant threatening effects on organisms like bacteria and marine life, which lead to waterways becoming toxic and leaching. In regard to ash and tobacco fertilisation, the study carried out by Wang et al.28 found that the results were positive. Tobacco is a natural product, so the results were positive. In combination with ash, it provides sufficient nutrients to enhance the growth of crops like barley (a historic agricultural practice).
A study examined how wood ash (a by-product of wood incineration) helped stimulate plant growth in Deschampsia flexuosa (wavey hair grass) when combined with pH increases, nitrogen and nutrient mineralization. As a general rule, wood ash preserves plant nutrients like phosphorus and potassium. However, most nitrogen molecules are lost to heat during the process. There is probably a small amount of nitrogen molecules bound to the residue, which prevents plants from using it. The high alkaline content of wood ash (pH > 12) results in a high concentration of metal oxides (Cd), which are potentially harmful if recycled or bioaccumulated. It is possible that roots of plants can absorb Cd, which can then be transported to the plant's surface. Generally, Cd in soil has a low value, and its value decreases with increasing pH.29 According to Anglia Ruskin University's Dr. Dannielle Green, cigarette butts reduce germination success and shoot length for some plants. Researchers examined the effects of smoked and unsmoked CBs on perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) in the greenhouse.30 After 3 weeks, both plants had reduced germination and shoot length due to CB exposure. Based on their findings, Green et al.,30 reported that measurable measurements have no effect on grass biomass. Clover roots and root-to-shoot ratios decreased after exposure to CB filters and remnant tobacco, however. Citing Green et al.,30 clover shoots showed an increase in chlorophyll-a, while grass shoots showed an increase in chlorophyll-b.
This study examined soil properties, seed germination, and seedling growth in relation to cigarette butts. As plant species, peas (Pisum sativum) and barley (Hordeum vulgare) were used in the experiment.
Cigarette butt collection and preparation
There have been several studies on the lethal impacts of butt leachates in aquatic environments.31-33 Therefore, the safety protocol provided by WHO34 was strictly followed when collecting and preparing the butts. CBs were collected from large wooden sand ashtrays at the entrances of two shopping malls. Afterward, the butts and unsmoked cigarettes, along with the ash, were dried in the sun for five days. A total of 300 hundred cigarette butts were ground into particles. When the soil was treated, it was contaminated with particles of butts, surrounding trapping papers, unsmoked cigarette remains, and burnt residues of ash.
Seed collection, soil preparation and experimental design
The seeds for spinach and barley were purchased from local garden and grocery stores. A total of 20 seeds were sown per vessel for P. sativum, and 30 seeds were sown per vessel for H. vulgare. In order to support germination and growth, all culture vessels were irrigated twice a week. The experiment was conducted in a glasshouse at the Melbourne Polytechnic Epping Campus, Victoria, Australia. In order to maintain a temperature of 20°C during the experiment, the glasshouse was equipped with heaters under the bench seating. This experiment used twelve rectangular plastic containers (8 liters, polyethylene terephalate). Six containers were used for P. sativum, and six containers for H. vulgare. Each container contained 3 kg of homogenised, nonsterile normal garden soil. For each species of plant, three treatments and three controls were designed. Therefore, each of the six containers was treated with 5 gm of cigarette butts mixture, labelled as treatment, and numbered. Six other containers contained homogenised, nonsterile normal garden soil labelled and numbered as controls. Before seeding both species, soil analysis was conducted. In order to conduct soil analysis, the soil was sent to a NATA accredited soil testing laboratory 'Nutrient Advantage' located in Werribee, Victoria. During the experiment, soil samples were collected twice, one week before and after the seeds were sown. The constituents of soil were compiled.
Measurement of biological responses: germination success, plant growth and biomass
To determine the effects of cigarette butts on seed germination, the timings of seed germination in experimental plots were observed. Data were collected and analyzed statistically to determine the percentage of seeds that germinated.
The number of germinated plants and the length (cm) of shoots were measured throughout the 6-weeks experiment. Using a ruler, we measured the length of plants at 5 random spots in each container and averaged it. To remove all plant material from the surface, P. sativum and H. vulgare shoots were harvested using fine secateurs on the final day of the experiment before any further manipulations were made. A total of fresh aboveground plant biomass was recorded.
To remove any adhering soil particles, plant roots were carefully removed from the soil matrix by hand and washed by hand. A gravimetric method was used to determine the biomass of fresh roots. The roots were then oven-dried at 65°C to determine their dry biomass. Using Selbys Scientific's 30L Laboratory Vacuum Oven, samples were dried at 65oC for 24 hours. In order to analyse the data further, all the data were tabulated.
Statistical analysis
All statistical analyses were conducted using R (R Team, 2024). All response variables were tested for normality using Shapiro-Wilkinson tests. When the distribution is approximately normal (Shapiro-Wilk test) - the assumption is not met, a Kruskal-Wallis rank-sum test (non-parametric test) has been run. An F-test for equality of variance does not meet the assumption of equality of variance (corrected in a t-test). In the case of dependent variables, t-tests and Kruskal-Wallis rank sum tests were used to compare independent variables (Control, Treatment).
Soil Characteristics
CB treatment significantly increased most chemical properties, but significantly decreased NO3-N and K. In addition, the CB treated soil was found to have an increase in ammonia nitrogen deficiency (Table 1).
|
Parameters |
Pre-treatment |
Post-treatment |
||
|
Control |
Treatment |
Control |
Treatment |
|
|
pH (1:5 Water) |
7.5 |
7.7 |
7.3 |
7.9 |
|
pH (1:5 CaCl2) |
6.9 |
7.2 |
7 |
7.3 |
|
Electrical Conductivity (1:5 Water) dS/m |
0.26 |
0.31 |
2.88 |
0.4 |
|
Chloride (mg/kg) |
90 |
150 |
170 |
250 |
|
Nitrate Nitrogen (mg/kg) |
30 |
32 |
1,800.00 |
17 |
|
Ammonium Nitrogen (mg/kg) |
2.7 |
3.2 |
110 |
3 |
|
Phosphorus (Colwell) (mg/kg) |
250 |
240 |
320 |
230 |
|
Phosphorus Buffer Index |
65 |
79 |
82 |
84 |
|
Sulphur (KCl40) (mg/kg) |
41 |
63 |
73 |
53 |
|
Cation Exchange Capacity (CEC) (cmol(+)/kg) |
18.2 |
20.8 |
28.1 |
20.4 |
|
Calcium (ammonium-acetate) (cmol(+)/kg) |
12 |
14 |
12 |
14 |
|
Magnesium (ammonium-acetate) (cmol(+)/kg) |
3.4 |
3.9 |
3.7 |
3.9 |
|
Sodium (ammonium-acetate) (cmol(+)/kg) |
0.46 |
0.5 |
5.3 |
0.87 |
|
Potassium (ammonium-acetate) (cmol(+)/kg) |
1.8 |
2.3 |
7 |
2 |
|
Available Potassium (mg/kg) |
690 |
900 |
2,700 |
790 |
|
Aluminium (KLC) (cmol(+)/kg) |
<0.1 |
<0.1 |
<0.1 |
<0.1 |
|
Aluminium % of Cations |
<1.0 |
<1.0 |
<1.0 |
<1.0 |
|
Calcium % of Cations |
69 |
67 |
43 |
67 |
|
Magnesium % of Cations |
19 |
19 |
13 |
19 |
|
Sodium % of Cations |
2.5 |
2.4 |
19 |
4.3 |
|
Potassium % of cations |
9.8 |
11 |
25 |
9.9 |
|
Calcium/ Magnesium Ratio |
3.5 |
3.6 |
3.2 |
3.6 |
|
|
|
|
|
|
Table 1 Chemical characteristics of the soil at the beginning and at the end of the experiment
Germination success
There was a 58% (control) germination rate and a 77% (treatment) germination rate for sixty peas (Figure 1). Germination rates of 65.5% were observed for barley seedlings grown in control culture vessels containing sixty seeds. Those in the treatment culture vessels on the other hand, exhibited a germination rate of 80%.
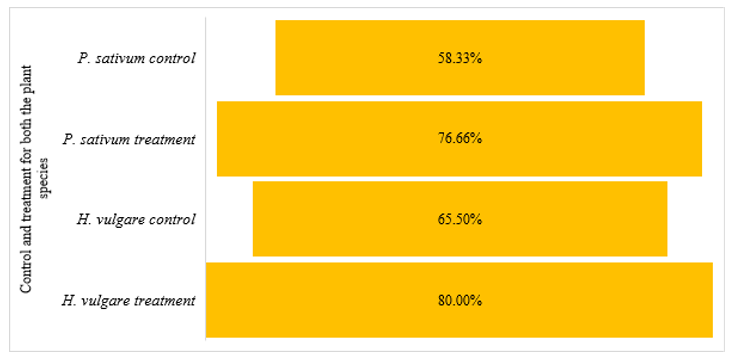
Figure 1 Seed germination of both the pea (P. sativum) and barley (H. vulgare) seeds in the control and treatment.
Plant growth and biomass
Barley growth performance measurements are illustrated in three figures: Figure 2, Figure 3, and Figure 4. Between treatment and control, there were significant differences in shoot height (n = 18, p= 0.0867), root height (n = 18, p= 0.8742), leaves length (n = 18, p= 0.01358), and total length (n = 18, p= 0.02088) (figure 2).
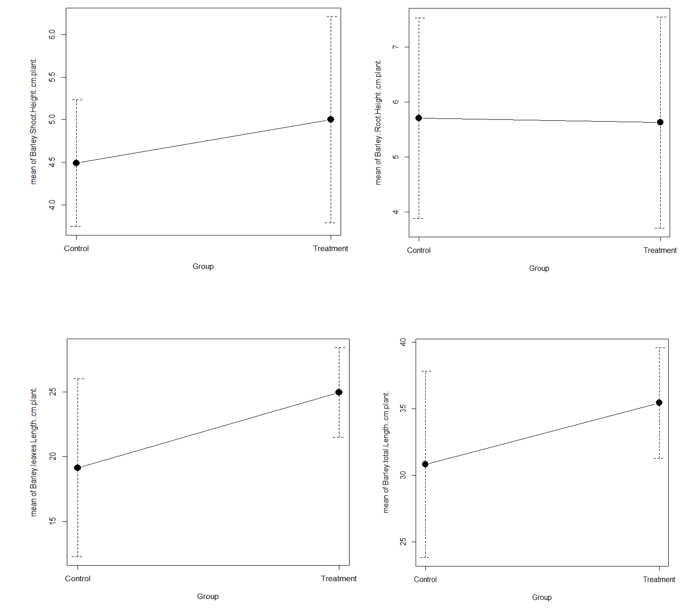
Figure 2 Barley shoot height, root height, leaf length, and total plant length in controls and treatments.
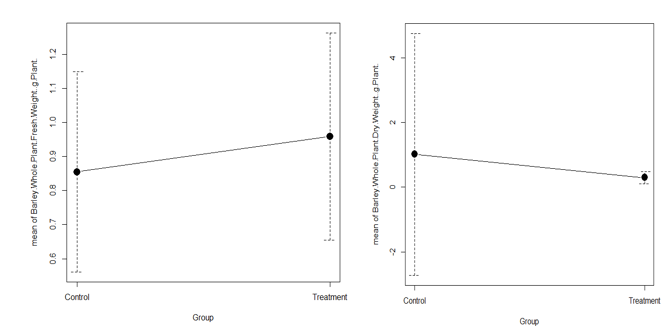
Figure 4 Weights of fresh and dry whole barley plants in treatment and control. Every week, the growth of barley in the treatment was higher than that of the control (Figure 5).
Weights of fresh and dry stems (P= 0.0923; P= 0.9166) and leaves (P=0.0093; P=0.0305) were also significantly higher in the treatment than control (Figure 3).
The fresh weight of the whole plant was significantly higher in the treatment (n=18, P=0.2545). Although, whole plant dry weight decreased in treatment (n=18, P=0.0038) (Figure 4).
Every week, the growth of barley in the treatment was higher than that of the control (Figure 5).
A similar measurement of peas growth performance is shown in three figures: Figures 6, and 7. There were significant differences in total length (n=18; P=0.0003) shoot height (n = 18, p= 0.0000), and whole plant fresh weight (P=0.0023) between treatment and control (figure 6). However, the whole plant dry weight (P=0.11589) was higher in the control group. This lower weight in treatment has an explanation.
In the treatment group, the fresh weights of the pea leaves were significantly higher than in the control group (Figure 7). The dry weight of the leaves was lower for peas treated with CBs, however.
A comparison of weekly growth of the control and the treatment in figure 8 shows that pea growth was greater in the treatment each week.
The soil chemical characteristics showed that pH, NO3-N, NH4-N, P Colwell, PBI Colwell, available K, calcium (amm-acet), magnesium (amm-acet) and calcium-to-mg ratio were similar between the day 0 control and day 0 treatment. Based on the pH values of weeks one and five, nutrients were available within the same growth range for both peas and barley. A slight change in other parameters, such as calcium (amm-acet.), magnesium (amm-acet.) and the calcium/magnesium ratio, was also observed. At week 5, Nitrate (NO3-N) and Ammonium (NH4-N) levels significantly increased in comparison to the controls, which is outside the optimal range.
It is widely acknowledged that both biodegradable and non-biodegradable cigarette butts have negative effects on bacterial communities when they leach certain elements into soils.6 Whenever cigarettes butts (CBs) come into contact with moisture, they release chemicals that lead to soil contamination. Ajibade et al.,35 claim that chemicals are released from cigarette butts (CBs).
According to several studies,36,37 cellulose acetate fibres are the most common material used in cigarette filters. It has been found that cellulose acetate, a bio-based plastic, has adverse effects on plants (including crimson clover, Trifolium incarnatum; Maramorosch,38 barley, Hordeum vulgare Chada,39 cucumber, Cucumis sativus Krizek and Mirecki,40 broad bean, Vicia faba, alfalfa, Medicago sativa Kieckhefer and Medler.41 The leachate and nicotine of cigarette butt are toxic to microbes, plants, benthic organisms, bivalves, zooplankton, fish, and mammals; however, there are critical knowledge gaps regarding the environmental impacts of tobacco product waste on ecosystems and environmental health.
A study by Green et al.30 found that while perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) germinated within three weeks, CBs reduced growth and did not affect grass biomass. While CB filters increased root-to-shoot ratios in clover, they decreased root-to-shoot ratios in other plants. This study used all the CB, whereas the one using only the ash and paper from the CB had an important difference. Since the cigarette filters had been removed from the paper casings, the treatment culture vessels contained only natural products, such as tobacco and paper.
As a result of this study, CBs treated soil showed higher germination and growth rates for Hordeum vulgare and Pisum sativum seeds. It has been demonstrated in numerous literature studies that every aspect of smoking cigarettes, from smoke to tobacco42 to leachate to the discarded filter and its associated leachate, can affect the health and productivity of plants.
As shown in figure 2, the heights of pea and barley plants were measured each week. Over time, peas grew significantly larger and faster than barley controls, but barley controls grew somewhat faster. Over the five weeks, there was almost no difference in the duration of the barley treatments. Peas and barley seedling growth and germination are not species-specific, since both germinated and grew into seedlings, proving that cigarette smoke affects soil structure and seedling growth.
Cheng et al.,26 found that here's an allelopathic inhibitory effect of nicotine when applied to seeds, and a hormesis effect when applied to germinated seeds and seedlings, which has an enhancement effect of 100 mg kg 1 on germinated seeds, and an enhancement effect of 200 mg kg 1 on seedlings under nicotine stress.
As tobacco is a plant product, and ash has been shown to enhance plant growth in previous research studies,29 this plausible explanation is that the treatment vessels resulted in increased growth and reflected fertilized soil in the soil sample results. It was demonstrated that Spanish broom (Spartium junceum L.), Lavender (Lavandula angustifolia) can be successfully established and grown in recycled cellulose acetate filters (CAF) in CB.43
As a general rule, plant stubble remaining from harvested crops can be burned to ash, in controlled conditions, to provide macronutrients and micronutrients needed for plant growth to the soil, including nitrogen, phosphorus, and potassium. It is necessary to conduct further research on whether cigarette filters, either individually or when mixed with paper casings and ash, will promote seed germination and seedling growth, as well as whether (at different toxicity levels they will affect seed germination, seedling growth and soil chemical properties.
According to Patel et al.11 and Wilson et al.,12 most urban smokers litter their used cigarette filters rather than dispose of them in trash cans. According to Rath et al.,44 this may be due to smokers not viewing cigarette filters as litter. It is therefore crucial to raise awareness that, even if cigarette filters are biodegradable, they can remain in the environment for years, and perhaps decades, even if they are biodegradable. During this time, they can have harmful ecological effects by reducing the growth and biomass of economically important primary producers with potential cascading effects on ecosystems.
Mohamed,45 indicated that cigarette butts able to affect the soil physico-chemical properties, where they increased the pH, EC & Soil alkalinity in tested soil. Sodium concentration were increased from 11.9 to 103.0 mg/kg.
Cigarette butts (CBs) have only recently begun to be considered environmentally harmful waste. CBs are common waste in the environment, that can cause air, soil, and water pollution and pose a threat to the living. CBs should be treated as toxic and hazardous waste due to its slow decomposition and accumulation of many toxic substances. There is a lack of research on the adaptation of CBs to the environment and what impact they have on vegetation.46
It is also evident that low concentrations of toxic substances contained in CBs have a positive effect on plants. This research shows that varied plant species can cope with different levels of contamination by hazardous elements.46 As cigarette smoke application increased, the plant height, the root length, the leaf width and length, the stem height, the chlorophyll a and b content, the guard cell length of wheat decreased.47
Several studies have documented the effects of CBs in soil and cigarette smoke on plant processes.48 Montalvão et al.49 found that the smoked CB leachate had cytotoxic, genotoxic, and mutagenic effects on onion (Allium cepa) roots at environmental concentrations (1.9 g/L of nicotine). Discarded CBs reduced the germination success and shoot length after 21 days of both perennial ryegrass (Lolium perenne) and white clover (Trifolium repens).30 These researchers suggested that their study demonstrates the potential for littered CBs to reduce the net primary productivity of terrestrial plants while da Silveira Fleck et al.50 reported elevated levels of metals in plants (Eugenia uniflora and Tradescantia pallida) near a designated outdoor smoking area, suggesting that SHS can result in the contamination of nearby flora. Noble51 found a universal decrease in the germination rate of radish (Raphanus raphanistrum subsp. Sativus), kale (Brassica oleracea), lettuce (Lactuca sativa L.), amaranth (Amaranthus spp.), wheat (Triticum spp.), rice (Oryza spp.), barley (Hordeum vulgare L.), and rye (Secale cereale L.) seeds when exposed to tobacco smoke.51 This negative response was not due to the presence of nicotine in the smoke, but rather to other non-volatile components. In contrast, Tileklioglu et al.47 reported that tobacco smoke increased the biomass of wheat and duckweed (Lemna minor L.) plants,47 and Mondal et al.42 found relatively little effect of tobacco smoke on the germination rate of Bengal gram (Cicer arietinum L.). Metal accumulation in plants is a common phenomenon and can affect humans indirectly by lowering plant nutritional value and directly through consumption of contaminated crops, even at low levels via chronic exposure.52
In an experiment, Wang et al.,28 recorded higher root biomass was in phyto-ash treatment than the control. Phyto-ash addition markedly influenced soil properties. Due to high pH of 8.6 in phyto-ash, application of 2‰ phyto-ash increased soil pH from 5.94 in control (fertilizer treatment) to about 6.35 in T1 and T2 treatments, consistent with the previous results.53 And significant increment of available K was observed in T2 treatment mainly due to more K from phyto-ash addition (73 mg kg_ 1) than the control and T1 treatments. Similar contents of organic matter, hydrolysable N, available P and exchangeable Ca and Mg were found among three treatments.
The fresh weight of barley and pea leaves in this experiment was significantly higher than that of the control. However, the dry weights of both were significantly lower than the controls. Thus, the treated plants retained more moisture than the control plants. There is no doubt that this moisture came from the soil. The soil treated with CBs can retain more moisture, which may be beneficial to plants. As part of this experiment, the whole cigarette butts were used, which means that the whole cigarette butts include both the filter and the little smoked cigarette portion, plus the ash on the tip. As a result, in the soils treated with cigarettes butts, there were three components: filter, tobacco, and ash. It is argued in this study that ash and tobacco parts of cigarettes affect soil positively. Additionally, it was not impossible that the filter part could also play an important role during germination. A filter can increase the moisture-holding capacity of soils by making them porous. It takes longer for the cellulose acetate in the filter to breakdown into microplastic. As a result of this short period of experiment, all elements in smoked cigarettes were beneficial to germination and growth. In spite of this, cigarette butts have detrimental effects on soil, biota, water quality, and the environment in general. There must be some negative effects on the plant, during germination and on seedlings. As far as similar experiments are concerned, most of them have been performed with just cigarette butts filters. In this experiment, however, the whole cigarette butt was used, including its ash and unburned cigarette contents. It appears that ash and unsmoked parts can provide nutrients to plants until they are reincorporated into the soil. It is therefore most likely that the filter parts of butts will affect soil health and plant nutrition after the ash and unsmoked butt tobacco contents have been depleted of nutrient sources. It will also take some time for the filter parts, which are actually cellulose acetate fibre, to mix with the soil. Therefore, it is difficult to determine the exact effect of cigarettes butts on seedling germination and growth in this short period of time. It is therefore suggested that the cigarette buts be separated into the three mentioned elements, namely tobacco, ash, and filter, in order to carry out this type of experiment. In order to conduct an experiment, two treatments must be designed, one with tobacco and ash and the other with only the filter. Therefore, these authors believe that the experiments can provide a better understanding of how cigarettes affect plants and the environment.
In light of these findings, cigarette butt research as well as seedling growth in peas and barley has provided a baseline for future research, as long-term effects remain uncertain. The discarded butts in soil may adversely affect future generations and the food supply they provide as a result of an increasing smoking population. This investigation appears to have been fruitful, laying the groundwork for more comprehensive studies on the environmental and health effects of cigarette litter in Australia and around the world.
It is with gratitude that the authors acknowledge the Head of the program and colleagues from the Department of Arts, Education, and AgriTech at Melbourne Polytechnic. A special thanks goes to Melissa Jackson who helped tremendously with the statistics.
In this research, there is no conflict of interest.

©2025 Taylor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.