eISSN: 2576-4462


Research Article Volume 9 Issue 2
1Environmental Microbiology Laboratory, Research Institute in Chemistry and Biology, B. B-3, University City, Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, México
2FES, Zaragoza. Universidad Nacional Autónoma de México Av. Guelatao 66, Ejercito de Oriente, INDECO, ISSSTE, Iztapalapa, 09320, Ciudad de México, CDMX, México
3Laboratory of Biotechnology and Biomonitoring of the Environment and Oasis Ecosystems (LBBEOE), Faculty of Sciences of Gafsa, University Campus of Ahmed Zarroug, University of Gafsa, Tunisia
4Laboratory of Bioresources, Biotechnology, Ethnopharmacology and Health Mohammed First University Faculty of Sciences, Morocco
5Translational Research Institute. Dr. Tetsuya Ogura Fuji, AC, Morelia, Mich., Mexico
Correspondence: Juan Manuel Sánchez-Yáñez, Environmental Microbiology Laboratory, Research Institute in Chemistry and Biology, B. B-3. University City, Universidad Michoacana de San Nicolás de Hidalgo, Francisco J Mujica S/N, Col. Felicitas del Rio, ZP 58030, Morelia, Michoacán, México
Received: April 14, 2025 | Published: April 29, 2025
Citation: Ordoñez-Juárez M, Cruz JLII, Pérez-González DA, et al. Seed inoculation with Methylobacterium symbioticum enhances growth of Phaseolus vulgaris under reduced NH4NO3 fertilization. Horticult Int J. 2025;9(2):75-80. DOI: 10.15406/hij.2025.09.00326
The healthy growth of Phaseolus vulgaris relies on fertilization of nitrogen with NH4NO3. However, excessive application leads to environmental degradation, including loss of soil fertility and, green gas emissions such as N2O. As an ecological alternative, this study investigates whether reduce the dose to 50% and inoculate P. vulgaris with Methylobacterium symbioticum, an endophytic bacterium can promotes plant growth. The objective of this research was to analyze the effect of M. symbioticum on the growth of P. vulgaris plus NH4NO3 at 50%. Three isolates of M. symbioticum, obtained from P. vulgaris leaves, were individually and jointly inoculated into bean seeds. A randomized block design was used with two controls (uninoculated plants with either 100% NH₄NO₃ or water only) and four treatments (three individual isolates and one combined treatment) under 50% NH₄NO₃ fertilization. Growth performance was assessed through germination rate, plant height, root lenght, and biomass at seedling and pre-flowering stages. Data were analyzed using ANOVA and Tukey’s HSD (p < 0.05).
The results showed a positive effect of M. symbioticum on the germination of P. vulgaris compared to uninoculated P. vulgaris and 100% NH4NO3. Likewise, a positive effect was observed on the phenology and biomass of P. vulgaris in the different isolates of M. symbioticum with 50% NH4NO3. There was evidence that although M. symbioticum is an endophyte of leaves of domestic plants, it can invade the root tissue of P. vulgaris. These findings suggest that inoculation with M. symbioticum allows for reduced nitrogen fertilization without compromising plant health, potentially mitigating environmental harm such as N₂O release and water contamination.
Keywords: soil, legume seeds, greenhouse gases, foliar endophyte, radical colonization, plant health
Phaseolus vulgaris (bean) is the second most produced and consumed crop worldwide, especially in Mexico, associated to beneficial endophytic bacterial genus and specie of Methylobacterium symbioticum.1-3 Its healthy growth is highly dependent on nitrogen fertilization, since M. symbioticum generates phytohormones from the organic compounds of the P. vulgaris root that improve and optimize the uptake of NH4NO3 reduced to 50%.4,5 However, the nitrogen fertilizer as NH4NO3 is not normally applied in doses according to the real nutritional need by plant5,6 but rather in excess, that causes loss of soil organic matter, decrease in microbial diversity, contamination of surface water or aquifers and the release of greenhouse gases.7 Due to the soil microbiota in anaerobiosis transforms NO3 into N2O which favors global warming with negative consequences for life on Earth,8 including able to oxide methane (CH4) a promising biological tool against global warming.9 An alternative solution is to regulate or reduce the dose of NH4NO3 without compromising the healthy growth of P. vulgaris, by inoculating the seed with Methylobacterium symbioticum, a genus and species of endophyte that, in addition to colonizing the leaves of P. vulgaris,10,11 also invades the roots of the legume in order to optimize the uptake of the reduced NH4NO3.12,13 Since foliar inoculation with P. vulgaris would entail a greater risk of a positive response,13 the objective of this work was to analyze the effect of M. symbioticum on P. vulgaris seeds plus NH4NO3 at 50%.
Soil collection and preparation
Agricultural non-sterile soil was collected from a site located at “La Cajita” belonging to the Zapata of the municipality of Morelia, Mich, México (19º 39’ 27’’ N 100º 19’ 59’’ W), with an altitude of 1820 m above sea level, with a temperate climate in an agricultural land . The texture of this soil was classified as clay loam, organic matter of 4.57% and a slightly acidic pH of 6.64. The soil was solarized at 70 °C/48 h to minimize pest and disease problems, then it was sifted with a No. 20 mesh. 1.5 kg of soil was placed on top of the Leonard jar, while the mineral solution or water in the reservoir at the bottom, both parts were connected by a cotton strip approximately 20 cm long to allow the movement of the solution by capillarity to the soil were P. vulgaris provided by the Secretary of Environment and Natural Resources of the Government of Mexico; seeds were sown as is shown in Figure 1.
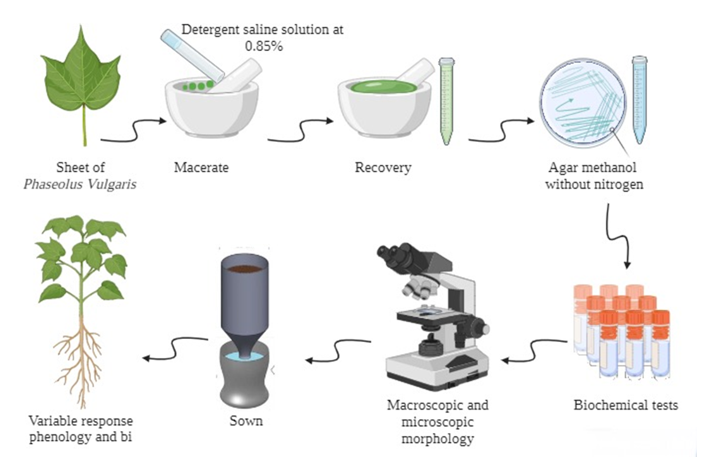
Figure 1 Isolation and inoculation of Methylobacterium symbioticum in Phaseolus vulgaris plus NH4NO3 at 50% The diagram of the materials and methods was generated by this laboratory, or Environmental Microbiology Laboratory-UMSNH.
Isolation of M. symbioticum from leaves of P. vulgaris
All M. symbioticum were isolate from the leaves of P. vulgaris, disinfected with 3% NaClO/5 min, rinsed 6 times with sterile water, then the root was disinfected, with 70% alcohol/5 min, rinsed 6 times with sterile water. Finally leaves were macerated, in a sterile mortar with 0.85% saline solution (NaCl), and 0.1% 123 detergent, sown with bacteriological loop, on methanol agar nitrogen free following chemical composition (g/L): methanol (after sterilizing culture media) 0.1, yeast extract 0.1, MgSO4, 0.5; KCl, 0.5; K2HPO4, 1.0; FeSO4*7H2O, 0.001; bacteriological agar, 18.0, pH 7.0,14 as is shown in Figure 1.
Experimental design
The trial was carried out with a randomized block experimental design with 5 treatments and 5 repetitions (Table 1), with 2 controls: P. vulgaris uninoculated and irrigated with water or absolute control (AC), P. vulgaris uninoculated, fed with 100% NH4NO3 or relative control (RC) and the treatments P. vulgaris with M. symbitoicum1 fed with a mineral solution with the following chemical composition (g/L): NH4NO3 12.0; KH2PO4 3.0, K2HPO4 3.5, MgSO4 1.5, CaCl2 0.1, FeSO4 0.5 mL and 1.5 mL (g/L) of the trace element solution: H3BO3 2.86, ZnSO4*7 H2O 0.22, MnCl2*7 H2O 1.81 and K2MnO4 0.09 in 1000 mL of distilled water for plants, adjusted to pH 6.8, applied every 3 days for two months as is shown in Figure 1.15
|
Phaseolus vulgaris |
NH4NO3 |
Methylobacterium symbioticum 1 |
Methylobacterium symbioticum 2 |
Methylobacterium symbioticum 3 |
|
Absolute control (AC) water
|
- |
- |
- |
- |
|
Relative control (RC) NH4NO3 |
100% |
- |
- |
- |
|
T1 |
50% |
+ |
- |
- |
|
T2 |
50% |
- |
+ |
- |
|
T3 |
50% |
- |
- |
+ |
|
T4 |
50% |
+ |
+ |
+ |
Table 1 Experimental design to analyze the growth of Phaseolus vulgaris with Methylobacterium symbioticum and 50% NH4NO3.
Seed Inoculation of M. symbioticum
The diagram of the materials and methods was generated by this laboratory, or Environmental Microbiology Laboratory-UMSNH.
Data collection and statistical analysis
The following response variables were measured at seedling and pre-flowering stages: germination percentage; phenology: at seedling stage plant height (PH) and root length (RL); and biomass which includes aerial/ root fresh and dry weight (AFW/RFW), (ADW/RDW) (Figure 1).15,16 The experimental data were analyzed using one way analysis of variance (ANOVA) and mean differences were tested with Tukey HSD at a significance level of p < 0.05 using Statgraphics Centurion XVI.12 software.
Biochemical and physiological characteristics of isolates
This culture media was incubated at 30oC for 72 h, The N2-fixing capacity of Methylobacterium isolates was determined in a nitrogen-free mineral medium combined (g/L) MgSO₄·7H₂O .2, K₂HPO₄ 0.1, KH₂PO₄ 0.4, FeSO4 0.01, NaCl 0.1 with methanol 10.0 ml as only source of carbon and energy, 10 ml/L at 0.5% bromothymol blue solution, pH adjusted to 6.7 in 1000 ml of distilled water. For partial identification, the following were considered: pigment production and colony diameter, optimal growth temperature and pH, as well as the utilization of L-alanine, ribose, L-arabinose, sodium acetate as carbon and energy sources, and NO₃ reduction as is shown in Figure 1.4,8,10,11,17
Genetic identification of isolates of Methylobacterium
The genome of M. symbioticum isolates from P. vulgaris leaves was sequenced using the Wizard Genomic DNA Purification Kit (Promega) using the Illumina HiSeq 4500 platform (Macrogen). Using Rapid Annotation using Subsystem Technology or RAST,10,11 (complementary data not shown).
Table 2 and Figure 2 show that seed germination of P. vulgaris individually and in a mixture of M. symbioticum, germination was observed from 79.16% to 91.66%, due to the invasion of M. symbioticum in the seed, which was able to converted organic compounds associated with germination, into phytohormones since M. symbioticum has the genetic capacity, to recognize them and transform them into phytohormones.2,3,5 The numerical values of germination were statistically different compared to the germination percentage of P. vulgaris with 70.83% used as RC, that implies that with the 100% mineral solution the seed does not induce the maximum germination of the seed,16,19 in part due to a lack of sufficient phytohormone that induces and regulates it as M. symbioticum does. Although instead of inoculating the foliar part of P. vulgaris,1 the seeds were treated, which implies that M. symbioticum has the ability to invade not only the aerial part of the plant2 it also responds to the organic products of germination4-6 in addition to being a way to enter the conduction system of P. vulgaris.8 M. symbioticum was recovered from the leaves of P. vulgaris, which increases the possibilities of applying M. symbioticum to support the healthy growth of P. vulgaris based on a dose that is uptake effectively,13,14 at the same time minimizing N2O generation6,9 and other negative impacts to the soil such as the loss of organic matter and / or the contamination of surface water or aquifers,15,18 by excess of NH4NO3 not uptake by P. vulgaris.19

Figure 2 Effect of Methylobacterium symbioticum individually or in combination with other isolates
of M. symbioticum 1, M. symbioticum 2, M. symbioticum 3 on the germination of P. vulgaris fed
with 50% NH4NO3.
AC, absolute control; P. vulgaris uninoculated with M. symbioticum, irrigated only water
RC, Relative control; P. vulgaris uninoculated with M. symbioticum, fed 100% NH4NO3
T1, Treatment 1; P. vulgaris + M. symbioticum 1 + 50 % NH4NO3
T2, Treatment 2; P. vulgaris + M. symbioticum 2 + 50% NH4NO3
T3, Treatment 3; P. vulgaris + M. symbioticum 3 + 50 % NH4NO3
T4, Treatment 4; P. vulgaris +M. symbioticum 1 + M. symbioticum 2 + M. symbioticum 3 + 50% NH4NO3
|
Phaseolus vulgaris* |
Germination percentage (%) |
|
Absolute control (AC) water |
62.5c** |
|
Relative control (RC) NH4NO3 at 100% |
70.83b |
|
T1=M. symbioticum 1+ NH4NO3 at 50% |
91.66a |
|
T2=M. symbioticum 2+ NH4NO3 at 50% |
79.16b |
|
T3=M. symbioticum 3+ NH4NO3 at 50% |
83.33b |
|
T4=M. symbioticum 1+ 2+3 NH4NO3 at 50% |
83.33b |
Table 2 Effect of Methylobacterium symbioticum on the germination percentage of Phaseolus vulgaris with 50% NH4NO3.
*n=6, **Different letters indicate statistical difference according to ANOVA/Tukey HSD P<0.05%
Table 3 shows the phenology at pre-flowering stage of P. vulgaris with M. symbioticum 3 and 50% NH4NO3 where it reached 59.06 cm of plant height (PH) and 14.46 cm of root length (RL), with not statical difference registered with M. symbioticum isolates 1, 2 and with the mixture of the 3 whose numerical values were statistically different compared to 21.66 cm of PH and 7.75 cm of RL of P. vulgaris not inoculated with 100% NH4NO3 or relative control (RC). In relation to the biomass of P. vulgaris with M. symbioticum 1 NH4NO3 at 50% reached 3.63 g of aerial fresh weight (FAW), 0.92 g of root fresh weight (RFW), 0.41g of aerial dry weight (DAW), 0.066 g of root dry weight (DRW) similar to the effect of isolates 2,3 and the mixture of the 3 isolates. This fact supports that when M. symbioticum colonizes the roots of P. vulgaris it can also move to the stem and leaves1,2 since M. symbioticum, unlike other endophytes of P. vulgaris such as Rhizobium, only colonizes and locates in the root tissue.5 With the advantage that from derivatives of photosynthesis and the metabolism of the radical system it synthesizes a diversity of phytohormones,12-16 inducing a maximum uptake of NH4NO3 to ensure a balance in the plant which allows it to grow healthily.19-23 In most cases these numerical values were statistically different or equal to the same phenological or biomass variables of P. vulgaris, not inoculated with 100% NH4NO3 used as RC with values of 1.58 g of AFW, 0.63 g of RFW, 0.16 g of ADW, 0.052 g of RDW. This shows that the 100% dose of NH4NO3 is not uptake by the plant's root system,24-26 and no growth improvement was observed than with 50% NH4NO3. This result supports the strategy of reducing the dose to 50% of NH4NO3 by inoculation with M. symbioticum to optimize it and avoid soil deterioration and N2O formation.7,26
|
Phaseolus vulgaris* |
Plant height (cm) |
Radical lenght (cm) |
Total fresh weight (g) |
Total dry weight (g) |
||
|
|
Aerial |
Radical |
Aerial |
Radical |
||
|
(AC) absolute control water |
20.68c** |
7.58b |
1.55c 0.46c |
0.15c 0.039c |
||
|
(RC) relative control NH4NO3 at 100%
|
21.66b |
7.75b |
1.58c 0.63b |
0.16c 0.052b |
||
|
T1=M. symbioticum 1+ NH4NO3 at 50% |
68.93a |
11.81a |
3.63a 0.92a |
0.41a 0.066a |
||
|
T2=M. symbioticum 2+ NH4NO3 at 50% |
57.0a |
12.7a |
2.80b 0.91a |
0.31b 0.048b |
||
|
T3=M. symbioticum 3+ NH4NO3 at 50% |
59.06a |
14.46a |
2.75b 0.92a |
0.33b 0.051b |
||
|
T4=M. symbioticum 1+ 2+3 NH4NO3 at 50% |
60.0a |
12.53a |
2.89b 0.64b |
0.34b 0.043b |
||
Table 3 Effect of Methylobacterium symbioticum on the biomass phenology of Phaseolus vulgaris
*n=6, **Different letters indicate statistical difference according to ANOVA/Tukey HSD P<0.05%
+ Applied; - Non Applied.
Figure 3 shows the positive response of P. vulgaris to M. symbioticum in the number and green color of the leaves, since M. symbioticum colonizes the radical system which transformed organic compounds released by plant metabolism into phytohormones21,22 to increase the height of P. vulgaris. With an increase in the size of the plant as well as the greater number of dark green leaves indicative of the maximum synthesis of chlorophyll. In addition, through an action of phytohormones of M. symbioticum 3 accelerated the flowering stage and the density of the roots.28-31 In the same way, a positive effect on the healthy growth of P. vulgaris was observed by isolates 1, 2 and in combination with number 3; with 50% NH4NO3 with improved growth of the leaf and root system,32,33 despite the fact that P. vulgaris seeds were inoculated with M. symbioticum instead of the foliar route - the most common application in plants. Meanwhile, at the level of the root system of P. vulgaris, with M. symbioticum, the maximum uptake of 50% NH4NO3 was registered, generating a vigorous and healthy plant. This is due to the profile of phytohormones that M. symbioticum can transform within the root and leaf tissues that communicate through the vascular conduction system.16,35 As shown in Figure 3, compared with the number, color, and size of the leaves, the density of the root system of P. vulgaris not inoculated with 100% NH₄NO₃. It was evident that the uptake of 100% NH₄NO₃ was not the maximum, compared to the growth registered in P. vulgaris with M. symbioticum and 50% NH₄NO₃. This demonstrates the versatility of M. symbioticum as an endophyte of P. vulgaris, from leaves to roots and vice versa, without any problem.36 Based on the type of metabolism of M. sybioticum, it is possible to optimize the reduced dose of NH₄NO₃ to prevent the remaining NO3- from being transformed into N2O by soil microorganisms, and at the same time, M. symbioticum oxidizes CH₄ in the soil generated by microbial anaerobic activity a positive compensatory effect on mitigating global warming caused by intensive agriculture with unregulated doses of chemical fertilizers and other chemicals.36-39

Figure 3 Effect of Methylobacterium symbioticum individually or in combination with other isolates of M. symbioticum 1, M. symbioticum 2, M. symbioticum 3 on phenology of P. vulgaris fed with 50% NH4NO3 at pre-flowering stage.
AC, absolute control; P. vulgaris uninoculated with M. symbioticum, irrigated only water
RC, Relative control; P. vulgaris uninoculated with M. symbioticum, fed 100% NH4NO3
T1, Treatment 1; P. vulgaris + M. symbioticum 1 + 50 % NH4NO3
T2, Treatment 2; P. vulgaris + M. symbioticum 2 + 50% NH4NO3
T3, Treatment 3; P. vulgaris + M. symbioticum 3 + 50 % NH4NO3
T4, Treatment 4; P. vulgaris +M. symbioticum 1 + M. symbioticum 2 + M. symbioticum 3 + 50% NH4NO3
Biochemical and genetic identification of isolates of Methylobacterium from leaves of P. vulgaris.
M. symbioticum isolates from P. vulgaris leaves showed colonies exhibited characteristic size and the common pink pigment of this genus and species on trypticase soy agar.10,17 These isolates had an optimal growth pH range of 6.0 to 7.3 and a temperature range of 25 to 30°C acetylene reduction activity4,13 as evidence of their potential to fix N2 from the air (data not shown). The same way Methylobacterium species is its ability to utilize single-carbon substrates, such as methanol and other methylated compounds, as sole carbon and energy sources.10,11 All of them used L-alanine as a nitrogen source, reducing NO3 to NO2, and ribose, L-arabinose, and sodium acetate as the sole carbon and energy source.20,25 Biochemical behavioral profile of this genus and species, an endophyte of legume leaves such as P. vulgaris. The G+C DNA content of the isolates ranged from 66 to 69.1% molar, sufficient to be considered as members of the Methylobacterium genus and species symbioticum,11,36,37 from leaves of P. vulgaris.29
This research demonstrates that M. symbioticum, an endophyte isolated from the phyllosphere of P. vulgaris and inoculate it into the seed of P. vulgaris to can effectively enters the plant through the leaves starting the stage of germination also has the capacity to uptake the 50% dose of NH4NO3 and through conversion of compounds from the leaves and roots, promote healthy growth, with improved phenology and biomass than the 100% NH4NO3 dose treatment. With the advantage of reducing NH4NO3 to 50%, the decrease of soil organic matter and microbial diversity, the contamination of surface and groundwater. Especially for the release of N2O derived from the anaerobic activity of the soil when it is not efficiently uptake by the roots of P. vulgaris without inoculated with M. symbioticum and is applied at 100% NH4NO3 treatment. The biochemical and physiological profile of the Methylobacterium isolates from leaves of P. vulgaris, as well as molecular analysis, supported the idea that this is the symbioticum species, particularly enhanced by its ability to oxidize methanol and to reduce acetylene as evidence of biological N2 fixation.
To the Coordinación de Investigación Científica de la UMSNH “Aislamiento y selección de microorganismos endófitos promotores de crecimiento vegetal para la agricultura y biorrecuperación de suelos” from the Research Project (2025), Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, México. For the information and experiences of the project: "Field Test of a Living Biofertilizer for Crop Growth in Mexico" from Harvard University, Cambridge, Ma, USA (2024) with support of Rockefeller Fund. To Phytonutrimentos de México and BIONUTRA S, A de CV, Maravatío, Michoacán, México for the P. vulgaris seeds and verification of greenhouse tests. To Paulina Correa-Flores for its technical support.
The authors declare no conflicts of interest.

©2025 Ordoñez-Juárez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.