eISSN: 2373-6372


To determine the effects of luminal inhibition of starch digestion on parameters of weight gain and plasma lipid profiles, groups of obese male T2DM SHR/Ntul//-cp rats were fed a nutritionally complete diet containing 54% sucrose as the only carbohydrate (CHO) component (Control) or the same diet containing an α-glucosidase inhibitor (1,5 dideoxy-1,5-[(2-hydroxyethyl) imino]-D glucitol; miglitol), 150 mg/kg diet, ad libitum under standard housing conditions) for up to 8 weeks post weaning. Miglitol resulted in modest decreases in weight gain. At the end of the study, bloods were collected for assay of plasma cholesterol, and α- lipoprotein (LDL) and β- lipoprotein (HDL) fractions determined. The miglitol-associated luminal inhibition of glucosidase activity resulted in 20% reduction in total cholesterol, and in both α- (LDL) and β-lipoprotein (HDL) fractions. These results indicate that simple inhibition of luminal α-glucosidase activity via miglitol may be a useful adjunct in the clinical management of hypercholesterolemia in states of obesity, T2DM and other glucose intolerant states, in addition to therapeutic applications in enhancing and improving glycemic control in man and animals.
Keywords: Obesity, Diabetes, T2DM, Cholesterol, Lipid Profiles, Sucrase, a-Glucosidase Inhibition, Miglitol, Rats
The current prevalence of obesity, type 2 diabetes and their common pathophysiologic sequalae are now approaching epidemic proportions in much of Westernized society, with no clear therapeutic resolution on the horizon.1 Elevations in plasma lipid profiles including total cholesterol (TC) and LDL Cholesterol (LDL-C) and modest decreases in HDL-Chol are among the most common observations in Obesity+T2DM, and represent a major contributor to a myriad of cardiovascular disorders often accompanying the condition.2 The hallmarks of most treatment regimens include modifying elements of diet, exercise and lifestyle, individually or in combination, and are often met with limited success. Dietary habits typically include both familial and cultural dietary practices and may be negatively influenced by economic and habitual constraints.3 In addition the adequacy and availability of nutritious foods may add an additional barrier to issues relating to food insecurity. Moreover, the recent influx of high fructose corn syrup (HFCS) sweeteners into the food supply chain has introduced yet another confounding constituent, resulting in a five-fold or greater increase in dietary fructose intake, and resulting in further pathophysiologic divergences in optimal metabolic pathways.4 Introduction of healthful dietary changes are often a challenge to implement, as the pathophysiologic changes thar may progress to more serious health issues typically occur gradually, and are often asymptomatic in the early stages of disease progression. Thus by the time their progression becomes physiologically apparent, the magnitude of disease has likely advanced, and more aggressive therapeutic measures may be necessary to arrest, resolve, or reverse further progression. Specifically, the process of atherogenesis often has an asymptomatic onset early in life, and while early stages of atherogenic changes may undergo reversal, as the disorder progress into adulthood the potential for effective reversal diminishes. Insulin resistance, a common observation in obese, T2DM states contributes to systemic inflammation, and to further progression of atherogenesis and its pathophysiologic sequelae.2
The pseudosaccharide compound 1,5 dideoxy-1,5-[(2-hydroxyethyl) imino]-D glucitol; miglitol; Glyset®) is an established competitive inhibitor of luminal starch digestion, where it binds to brush border glucosidase enzyme receptor domains, thereby delaying the rate of digestion and subsequent luminal glucose uptake from the gastrointestinal tract.5,6 Starches normally undergo rapid digestion into simple sugars in the upper echelons of the small intestine, where the rate-limiting step in glucose uptake approximates to the rate of glucosidase activity. Diet composition, especially the presence of dietary fibers, gums and pectins can impede the rate of luminal brush border digestion, albeit with some gastrointestinal distress when the relative contributions of the indigestible fibers exceed ideal proportions and/or gastrointestinal thresholds. As the rates of luminal CHO digestion become decreased, plasma insulin requirements may also plateau at a lower magnitude, as less insulin would then be required to facilitate monosaccharide uptake and disposal in peripheral tissues.7 The insulin-lowering phenomenon may be enhanced, since the haff-life of insulin receptor activity is typically considerably longer than that of starch digestion and subsequent monosaccharide oxidation. Thus, dietary supplements or additives that might extend the process of luminal digestion of starches and luminal absorption of simple carbohydrates pose an interesting prospect in modulating downstream physiologic plasma insulin activities, including the metabolic effects of insulin on lipogenic and cholesterol generating parameters. Insulin exerts numerous effects on multiple key parameters of intermediary metabolism, including modulation of protein turnover, carbohydrate oxidation and storage, and lipogenesis to cite just a few that are pertinent to this study. Thus, the purpose of the present investigation was to determine the effects of partial luminal α-glucosidase inhibition on plasma lipid profiles with miglitol, and were conducted in an animal model where early onset obesity, hyperinsulinemia, insulin resistance and T2DM occurs during early stages of adolescence and remains present thereafter.8-10 The obesity and progression to T2DM occurs via expression of an autosomal recessive epigenetic trait, accompanied soon afterward with the commonly observed progression of pathophysiologic sequelae including derangements in plasma cholesterol and lipid profiles.8,9
The SHR/Ntul//-cp rat model was developed in the small animal genetics unit at the NIH by Hansen by incorporating the -cp trait from the Koletsky rat into a longevity-prone NIH (N) strain of unknown origin.8,11 This was followed by crossing the N-cp strain with the spontaneously hypertensive rat (SHR), and completing 12 or more cycles of backcrossing sufficient to establish congenic status while preserving the SHR and -cp traits. The hypertensive trait was preserved only in the lean phenotype while the T2DM developed soon after weaning in the obese phenotype, and the newly developed SHR/N-cp strain preserved the albino coat of the SHR strain. Both phenotypes exhibit a significantly decreased lifespan due to complications of T2DM compared to their longevity-prone NIH (N) heritage.9
Groups of congenic obese male SHR/Ntul//-cp rats (n= 8 rats/group) housed under standard laboratory conditions of temperature (21-22 degrees C/ 50% RH) on a reverse light cycle (dark 0800-2000 daily) in adjacent hanging steel cages with individual occupancy. Animals were fed Purina Chow and house water ad libitum from weaning to 8 weeks of age, at which time early stages of obesity and T2DM were clearly established and glycosuria and T2DM confirmed. At 8 weeks of age, rats were switched to a semi-purified control diet developed at the Carbohydrate Nutrition Laboratories of the USDA that contained 54% carbohydrate as sucrose, 20% protein as equal parts casein and lactalbumin, 5.9 % cellulose, 16% fats as equal parts beef tallow, lard, corn oil, and hydrogenated coconut oil, 3.1% AIN vitamin salt mix, and 1% Teklad vitamin fortification mix (Control diet).10 The energy content of the diet was computed to provide 48.2 % of calories from CHO, 33.3 % of calories from fats, and 18.5% of calories from protein respectively as described elsewhere.10 The semi purified diet was fed ad libitum for up to 8 weeks duration. In addition, additional quantities of the control diet were fortified with 150 mg of the α-glucosidase inhibitor per kg diet (equal to ~ 2.5 mg of miglitol/rat/day) and also fed to the α-glucosidase inhibitor treatment group for up to 8 weeks duration. Body weights were monitored periodically throughout as an indicator of wellness. At the end of the study, rats were fasted overnight and blood obtained via tail bleeding in heparinized tubes for plasma lipid analysis. Plasma cholesterol and the α- lipoprotein and β-lipoprotein fractions corresponding to the LDL and HDL fractions respectively were determined spectrophotometrically following affinity chromatographic separation via the procedure of Bucolo and David.12 Data were analyzed via standard statistical procedures including application of Pages ‘L’ test for trend analysis where statistical significance via the ‘t’ test was suggestive but not confirmatory.13,14 The study was approved by the Institutional Animal care and Use Committee.
Initial and final Body weights and net weight gain of rats over 7 weeks of observations are depicted in Figure 1 and indicate that initial weights were similar in both treatment groups (263±11 g. vs. 263±12 g). The a-glucosidase inhibitor miglitol resulted in modestly (~13%) lower rates of weight gain and in similarly lower final body weights during the 7 weeks of observation of the study. (Control vs. Drug FBW: p. = < 0.05 via trend analysis; Control vs Net Gain: p=<0.05 via trend analysis.)
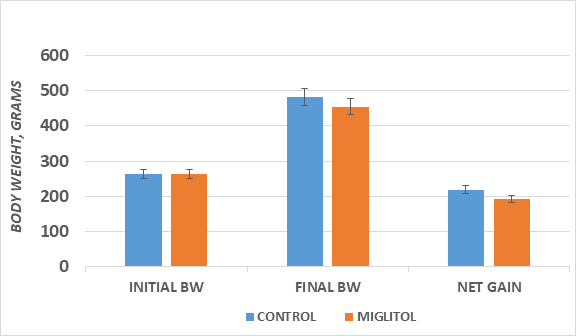
Figure 1 Effect of α-glucosidase inhibition on body weight of T2DM rats. Data are mean ± 1 SEM,
n= 8 rats/treatment group. Students ‘t’ test, and via Pages L test for trend analysis.
The effects of luminal α-glucoside inhibition on plasma total cholesterol are depicted in Figure 2 and indicate that α-glucosidase inhibition resulted in an approximate 20% decrease in total plasma cholesterol concentrations after <8 weeks of the dietary and pharmacologic treatment. In addition, the final concentrations of both the LDL and the HDL lipoprotein fractions were both decreased by an average of ~18-20% following the α-glucosidase treatment, and the effects were nearly evenly distributed across both LDL and HDL fractions. In addition, the lipoprotein ratios are depicted in Figure 3 and further indicate evidence that the pharmacologic treatment of luminal α-glucosidase activity with miglitol was without significant effect on lipoprotein ratios, thereby indicating that the effects of the anti-glucosidase agent were equally distributed across all lipoprotein fractions, consistent with a predicted global effect of improvements in insulin actions on lipid biosynthesis and metabolism.
Type-2 diabetes mellitus (T2DM) in association with obesity and overweight conditions is now one of the most prevalent metabolic disorders in the world and it prevalence is increasing at an alarming pace throughout Westernized society with more than a third of the population now impacted.1,3 This has placed an enormous burden on the available health care resources of many communities In addition to the economic losses in the workplace productivity due to decreased individual and collective capacity when individuals are unable to attend to their workplace obligations.1,2 Advances in the industrialization of food processing and distribution has also brought with it emerging changes in diet preferences and nutritional practices from those of past generations, and which have now inadvertently contributed to unsuccessful attempts to maintain energy balance in a more sedentary society. Accordingly, it is important to explore novel approaches to combat the emerging trends in disordered energy balance and their contributions to pathophysiologic sequelae which may result from the technological advancements. While no single strategy has yet been demonstrated to combat the emerging trends in the obesity+T2DM dilemma, several relevant animal models have been developed, including the Wistar Fatty Rat, the SHR/Ntul//-cp rat, the LA/Ntul//-cp rat, the Zucker fatty rat and others.8,9,15-18 These models may be applied to provide insight into effective environmental and pharmacologic strategies to address the issues and to further elucidate the pathophysiologic mechanisms involved.
The ingestion of high carbohydrate, high glycemic index diets common to Western society are typically contraindicated in Obesity+T2DM, where they are commonly associated with elevations weight gain, adiposity, and in increases in fasting plasma lipid profiles and other stigmata of obesity+T2DM.1,3 Therapeutic considerations include changes toward a more healthy life style, including diet planning that includes a complex carbohydrate, modest fat diet, with special attention to ensure adequacy in fiber and micronutrient intake, resulting in a lower glycemic index while more closely matching caloric intake to the projected energy requirements of the individual. The added incorporation of starch blocker agents such as acarbose, miglitol or other natural inhibitors of starch digestion may be additive to the luminal effects of the lower glycemic index complex carbohydrate diet regimen.3 Such a regimen may bring about sufficient weight loss with corresponding improvements in pathophysiologic stigmata, which may contribute to improvements in the metabolic profile of the individual. Luminal modulation of CHO digestion and monosaccharide absorption via glucosidase inhibitors combined with naturally occurring food components (often from vegetarian sources) may also bring about similar effects on α-glucosidase digestive activity.5
Miglitol is a complex oligosaccharide that acts as a competitive, reversible inhibitor of membrane-bound intestinal α-glucoside hydrolase activity and has been found to be a useful agent in treating mild to moderate severity T2DM.20 While luminal absorption of miglitol is complete or nearly complete at lower dosages, the luminal threshold for absorption within the first two hours is incomplete at dosages of 100 mg or more. The absorbed drug is normally excreted unchanged via renal mechanisms within a few hours of administration.37-40 In a previous animal study of 8 weeks duration with acarbose, an analog of miglitol, the HbA1c and glycemic responses typically demonstrated improvement toward normalization over time.6 The results of this study are consistent with previous clinical findings in the Wistar Fatty Rat, however, and confirm that the 8-week trial of feeding a highly palatable high carbohydrate sucrose-laden diet to obese adult Wistar Fatty Rats with well-established T2DM resulted in excess weight gain and which excursion was partially ameliorated by α-glucosidase inhibition. The obese phenotypes of the Wistar fatty rat and the SHR/Ntul//-cp rats both demonstrate significant insulin resistance, whether assessed by the fasting insulin to glucose ratio or application of the HOMA calculation, and which demonstrated significant improvement when offered the glucosidase inhibitor regimen including an attenuation of the hyperphagia commonly associated with the obese phenotype.6,7 in another study, Boque’ et al21 fed a nutritionally similar high carbohydrate regimen to a lean phenotype of male Wistar rats and also reported an increase in fasting triglyceride concentrations from the high CHO diet. In the present study, the miglitol treatment was associated with improvements in Total cholesterol, LDL-cholesterol and HDL cholesterol fractions, and an attenuation in the sucrose linked weight gain. Because only animals of the obese phenotype were included in this study, if was not possible to determine if the final weight profiles might have equated to those of their lean littermates when fed similar diets.
Whether the capacity of miglitol to effect a normalization of glycemic changes and in a more complete normalization of weight gain secondary to metabolic effects of pharmaceutically delayed carbohydrate digestion and subsequent luminal glucose absorption is unclear. If the favorable responses of miglitol administration may have been secondary to insulin lowering effects also remains unclear. However, the effects noted are likely a consequence of peripheral insulinogenic effects reflecting improved insulin sensitivity and insulin actions in insulin-dependent peripheral tissues including skeletal muscle and adipose tissue, major sources of peripheral insulin resistance. In contrast, the correction of long established hyperlipidemia of Obesity+T2DM likely may take a longer duration of treatment to return to pre-T2DM levels regardless of the therapeutic mode of treatment than was reported in the present study. Vedula et al noted that the pattern of food intake differed when the glucosidase inhibitor acarbose was fed as an admixture to lean and obese rats, thereby facilitating a comparable improvement in glycemic responses accompanied with similar improvements toward normalization of plasma lipid parameters.22
The lipid profiles in the obese+T2DM phenotype of this and other strains have been associated with increases in biochemical markers for free radical development and are clearly consistent with atherogenic lipid profiles, including elevations in both serum triglycerides and cholesterol, including the Low density (Alpha) lipoprotein fraction which have been associated with senescent alterations in the vascular intima.2 Although the lipid parameters were improved but not completely normalized in the obese+T2DM rats following the miglitol treatment, the excess weight gain of rats consuming the high sucrose diet was modestly improved by the miglitol, suggesting that the relatively short duration of the therapeutic regimen may have been at least partially effective in attenuating the hyperglycemic-hyperinsulinemic status. With some improvement in insulin sensitivity, the normalization of the lipid profiles may require a longer treatment duration or a greater dose of the α-glucosidase inhibitor agent. Thus, the use of α-glucosidase inhibitors for the treatment of glucose intolerant conditions remains a useful approach in controlling the insulin dependent hyperglycemic sequelae of the obese+T2DM phenotype of this strain and supports its usefulness in the treatment of T2DM in humans.22-31 While individual adipose depots were not examined in this study, other studies in this strain noted marked increases in mass and cellularity in the visceral retroperitoneal depot, along with smaller increases in other subcutaneous depots when fed a similar high sucrose diet. Thus, long term treatment with α-glucosidase inhibitors especially when combined with dietary intervention may bring about effective long-term improvements in both glycemic and lipid profiles, and in a likely decreased rate of progression of the pathophysiologic sequelae of T2DM and in atherogenic markers of cardiovascular disease and senescence associated with the deranged glycemic and lipid profiles. In contrast, while the effects of miglitol on lipid parameters have sometimes been variable, those differences are likely due to differences in animal models, patient populations, duration of pre-existing illness, dosages employed, and duration of treatment, the improvements are likely attributable to an improved economy of insulin sensitivity, glucose utilization and lipid metabolism and storage in perpheral tissues.
No discussion of potential side effects of luminal α-glucosidase or sucrase inhibitory agents would be complete without addressing potential side effects, most of which are generally dose-related, predictable and of limited duration.5,37-40 In animal studies, the agents are routinely administered as an admixture to a defined diet, while in clinical studies, the typical dosage forms are administered as tablets or caplets, taken at the onset of a meal, resulting in a more concentrated blend when introduced into the duodenum where luminal carbohydrate digestion commences. As a result, typical largely dose-related side effects of glucosidase and sucrase inhibitors may result in bloating cramping, abdominal distress, loose stools, and which may progress to flatulence likely due to undigested carbohydrate interacting with the colonic microbiota, where any residual carbohydrate can be metabolized. The colonic microbiota can readily digest and metabolize most carbohydrate sources, resulting in the formation of acidic metabolites and gas, adding to symptoms of abdominal distress. Because miglitol is absorbed into the systemic circulation but not metabolized via P-450 in the liver or other organs, a theoretically greater potential for allergic reactions may occur than with non-absorbable α-glucosidase inhibitors such as acarbose. Typical allergic symptoms from miglitol include hives or other allergic manifestations, and indicate immediate discontinuation of the agent, while the abdominal discomfort symptoms may often be attenuated by adjusting the dosage regimen. Additional side effects may include hypoglycemia, especially when administered in combination with insulin, sulfonylureas, or other hypoglycemic agents, due to apparent differential and additive glucose lowering mechanisms of combination therapy. In the present animal study with miglitol however, no adverse effects were observed with the dosage employed, and stool production and physical indicators of abdominal distress were remarkably uneventful.37-40
The short-term administration of a modest dosage of the α-glucosidase inhibitor miglitol in the presence of a high glycemic index sucrose-enriched diet was found to be useful in attenuating the excess weight gain and increases in plasma cholesterol, LDL-cholesterol, and HDL-cholesterol concentrations in the adult male obese SHR/Ntul//-cp Rats. These observations are consistent with typical dietary recommendations for consumption of complex carbohydrate, fiber rich diets for a variety of glucose-intolerant conditions., and suggest that miglitol when administered as a dietary admixture may be a useful therapeutic adjunct in the treatment of obesity+T2DM, and in an attenuation in the chronic systemic inflammation and pathophysiologic sequelae associated with the obese-diabetic state. Moreover, the clinical effectiveness of luminal glucosidase inhibitors on lipid parameters may be enhanced by the addition of a cholesterol lowering agent or other ttherapeutic adjunct, as has now been demonstrated in multiple clinical trials. The clinical efficacy of miglitol has now been demonstrated in numerous clinical trials and it’s demonstrated effectiveness in treating obesity associated T2DM is supported.32-37
The author thanks the Carbohydrate Nutrition Research Laboratory, of the US Dept of Agriculture, Beltsville, MD for the generous gift of dietary components used in this study.
The author reports no competing interests.
The author reports that no applications of AI were utilized in the generation of this manuscript.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.