eISSN: 2373-6372


Mini Review Volume 2 Issue 1
Department of Internal Medicine, Yale University School of Medicine, USA
Correspondence: Edwin C Thrower, Department of Internal Medicine, Yale University, LMP 1080, 333 Cedar Street, New Haven, Connecticut 06520, USA
Received: January 30, 2015 | Published: March 17, 2015
Citation: Patel V, Sreekumar B, Thrower EC (2015) New Insights into Cellular Mechanisms in Smoking-Related Acute Pancreatitis.Gastroenterol Hepatol Open Access 2(1):00028. DOI: 10.15406/ghoa.2015.02.00028
Pancreatitis is a painful inflammatory condition arising from pancreatic injury. Gallstone disease and alcohol abuse are the best known causes of pancreatitis, although more recently, cigarette smoking has emerged as a prominent risk factor. A wealth of clinical evidence underscores the role smoking plays in the disease, although knowledge of how cigarette toxins initiate pathological cellular events in the pancreas remains unclear. This mini-review focuses on recent research and highlights how cigarette smoke and smoking compounds may affect cell signaling pathways to initiate pancreatitis responses.
Keywords: pancreatitis, zymogen activation, nicotine, NNK, nicotinic acetylcholine receptors
NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; nAChR, nicotinic acetylcholine receptors; PSTI, pancreas-specific trypsin inhibitor; CCK, cholecystokinin; ET-1, endothelin-1
Pancreatitis is an inflammatory disease resulting from injury to the pancreas. A key initiating cellular event is premature activation and retention of digestive pro-enzymes (zymogens) in pancreatic acinar cells, resulting in auto digestion of the pancreas.1 This is followed by inflammation, edema, ischemia, and cell death through apoptosis or necrosis.2 Common etiologies include gallstone disease and alcohol, although numerous clinical studies now indicate that smoking alone can increase the risk of pancreatitis.3–6 Only recently has research delved into the molecular mechanisms that may underlie pancreatitis caused by cigarette smoke or one of its many components. A number of toxic chemicals are present in cigarettes but only nicotine and the tobacco-specific nitrosamine, 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) have been extensively studied with regards to pancreatic diseases”.7–14 Animal and cellular models have been developed to explore how these compounds initiate or sensitize the pancreas to disease and an overview of these findings are presented in this mini-review.
Models of cigarette-smoke induced pancreatitis
Cigarette smoke exposure
A study in which rats were exposed to 12 weeks of cigarette smoke resulted in development of pancreatic damage reminiscent of chronic pancreatitis. In addition to pancreatic damage, these rats had increased levels of digestive enzymes such as trypsin9 and altered gene expression of the endogenous trypsin inhibitor, PSTI (pancreas specific trypsin inhibitor).10 When this model included ethanol intake as an additional factor, two more indicators of pancreatitis, leukocyte infiltration and increased pancreatic ischemia were observed.12 Animal models that employ cigarette smoke exposure are useful in demonstrating effects of cigarette smoke on the pancreas, and development of pancreatitis responses. However they reveal little about which specific chemicals in cigarettes initiate these responses and the cellular mechanisms involved. In the subsequent sections, we describe studies which have focused on potent constituents of cigarette smoke: nicotine and its metabolite NNK.
Nicotine and NNK treatment
Nicotine: Nicotine is a key component of tobacco and cigarettes and likely contributes to development of pancreatitis and pancreatic cancer. Many rodent models exploring the effects of nicotine in pancreatic disease have been developed. Nicotine exposure in rats caused similar pathological changes in the pancreatic acinar cell as seen during acute pancreatitis. These included decreased enzyme secretion, pyknotic nuclei, cytoplasmic swelling and vacuolization.7 Figure 1 Furthermore, treating rats with radio labelled 3H-nicotine resulted in its accumulation in the pancreas.15,16 When nicotine exposure was combined with hormonal (cholecystokinin) stimulation in rats, decreased enzyme secretion and retention of zymogens within pancreatic acinar cells was observed.17–21 A more recent study showed that secretory effects induced by nicotine exposure in pancreatic acinar cells were abolished when the cells were treated with nicotinic acetylcholine receptor (nAChR) antagonists22 and calcium channel antagonists.23 These findings indicate that nicotine mediated pancreatic damage could be modulated by nicotinic receptors and calcium channels within pancreatic acinar cells.
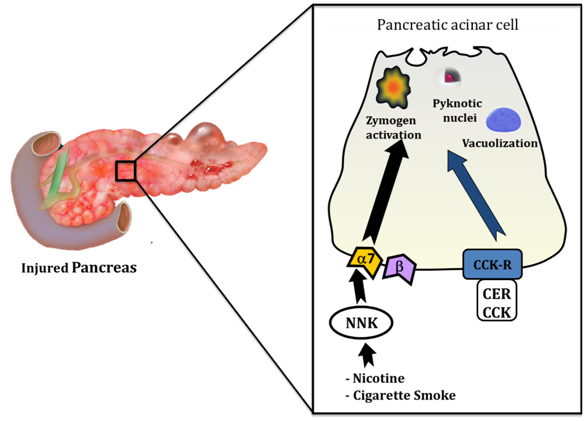
Figure 1Effects of cigarette smoke and its toxins in pancreatitis.
Toxins from cigarette smoke interact directly with the pancreatic acinar cell to induce pathological cellular changes consistent with those seen in pancreatitis. These changes include premature activation of digestive enzymes (zymogens), development of pyknotic nuclei and formation of large vacuoles within the cell. These cellular events can be initiated by cigarette toxin NNK via a non-neuronal α-7 nicotinic acetylcholine receptor (α-7) on the surface of the acinar cell. NNK may also mediate pancreatitis responses through a b-adrenergic receptor, but zymogen activation is not one of them. Cerulein (CER) is an orthologue of the hormone cholecystokinin (CCK) and is used to initiate experimental pancreatitis at supraphysiologic concentrations (10-100 nM) through the CCK receptor (CCK-R); NNK augments the effects of cerulein and indicates that cigarette toxins may also sensitize to pancreatitis.
NNK: NNK is a tobacco-specific nitrosamine, formed from nicotine, and is one of the most abundant and potent carcinogens in cigarettes and tobacco-related products.13 Prior studies of NNK and the pancreas have focused on its contribution to pancreatic cancer, but a role in initiation of pancreatitis has recently been described by Alexandre et al.24 In this study NNK exposure alone caused premature activation of digestive zymogens (trypsinogen and chymotrypsinogen) within pancreatic acinar cells.
Alexandre et al.24 also found that NNK pre-treatment could exacerbate responses in an established model of the disease. Cerulein, an orthologue of the hormone cholecystokinin (CCK), is given to isolated cells or animals in supraphysiologic concentrations (10-100x that required to induce physiological responses), to induce experimental pancreatitis. Pre-treatment of cells or animals with NNK in a cerulein model of the disease lead to elevated activation of zymogens above that seen with NNK or cerulein treatment alone. NNK was also shown to inflict cellular damage in the pancreas (vacuolization, pyknotic nuclei, and edema) after a two week treatment period in rats. Cumulatively, these data suggest that NNK can act as an initiator and a sensitizer to pancreatitis Figure 1.
By using their cellular and animal model approaches, Alexandre et al.24 were able to take their study one step further and explore underlying mechanisms of NNK-mediated pancreatitis. Those findings and other mechanistic studies are discussed further in section 6.
Cellular mechanisms involved in smoking related pancreatitis
In this section, particular attention is given to NNK-mediated mechanisms in smoking-related pancreatitis. This list is by no means exhaustive, but it does implicate signaling pathways which should receive consideration when developing disease treatments.
Nicotinic acetylcholine receptors
A previous study implicated nAChR as a potential target for nicotine-mediated effects in the pancreas.22 Nicotinic acetylcholine receptors (nAChRs) were initially characterized within the nervous system, but have subsequently been found in non-neuronal cells.22 NNK can bind with high affinity to nAChRs, particularly the α-7 isoform (α-7 nAChR). Alexandre et al.24 demonstrated the presence of non-neuronal α-7 nAChRs in rat pancreatic acini and showed that NNK induced zymogen activation was abrogated when cells were treated with the nAChR antagonist mecamylamine. These findings were further supported by studies in α-7 nAChR-/- mice where NNK treatment failed to elicit zymogen activation (unpublished observations, Dr. Edwin Thrower). This study constitutes the first to identify effects of cigarette toxins in pancreatitis through a direct effect on the acinar cell via a receptor-mediated mechanism. Whether α-7 nAChR potentiates other pancreatitis responses remains the focus for future research.
β-Adrenergic receptors
In addition to α-7 nAChR, another pathway through which NNK might potentiate pancreatitis is via β- adrenergic receptors, due to the structural similarity of NNK and β- adrenergic receptor agonists.25 Activation of these receptors can cause production of cyclic AMP through adenylate cyclase and 0this generation of cAMP can initiates or sensitize to pancreatitis responses.26 Although Alexandre et al.24 detected β- adrenergic receptors in rat acini, NNK mediated zymogen activation was not inhibited when β- adrenergic receptors were blocked. Whether these receptors are involved in other stages of pancreatitis remains to be determined.
Thiamin deficiency
Recent findings illuminate the role thiamin uptake may play in smoking-related pancreatitis.27 Protein and mRNA levels of thiamin transporters THTR-1 and THTR-2 were significantly reduced when pancreatic acinar 266-6 cells were treated with NNK. This reduction in thiamin transporters also reflected a decrease in thiamin uptake and thiamin transporter promoters- SLC19A2 and SLC19A3. Long term treatment of NNK in mice yielded similar results.27 This demonstrates that cigarette toxins can cause changes within the pancreas at a genetic level and thiamin deficiency, followed by reduction in cellular ATP levels, might sensitize the pancreas to a secondary insult leading to pancreatitis.
Endothelin-1
Another interesting recent discovery, although not mechanistic in nature, implicates Endothelin-1 (ET-1), a protein that plays a role in blood vessel constriction, in smoking related pancreatitis. Plasma ET-1 levels were nearly twice in smokers when compared to controls. Similar results were also seen between non-smokers and smokers suffering from chronic pancreatitis.28 Histopathological analysis of the pancreas also showed an increase in ET-1 levels in smokers and smokers suffering from chronic pancreatitis.28 These findings may account for changes in blood flow to the pancreas seen during pancreatitis and will require further mechanistic study.
Conclusions
Currently, little information regarding the pathogenesis of smoking-induced pancreatitis is available, especially when compared with other forms of the disease, including those linked to alcohol abuse and gallstones. Development and refinement of dependable animal models of smoking-related pancreatitis, in addition to human studies, will prove necessary if successful therapies are to be identified and utilized in disease treatment. Models concentrating on cigarette toxins prone to initiating human disease, such as nicotine and it’s more potent derivative NNK, have generated promising results. In particular, the α7nAChR warrants further attention as a potential therapeutic target, given its proposed contribution to premature digestive zymogen activation and other pancreatitis responses.24
Additional environmental and/or genetic factors may further promote pancreatic injury in combination with smoking. Studies designed to reveal how smoking alone, or in combination with these factors, mediates key pancreatic processes will greatly improve understanding of mechanisms underlying smoking-induced pancreatic diseases.
None.
The authors would like to acknowledge Connecticut Department of Public Health (DPH contract log # 2014-0138), National Institute on Alcohol Abuse and Alcoholism (R21 AA-020847-01) and the Department of Veterans Affairs for support.
The authors declare that they have no conflicts of interest.

©2015 Patel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.