eISSN: 2373-6372


Research Article Volume 16 Issue 2
1University Clinic of Hepato-gastroenterology, Hubert Koutoukou Maga National University Hospital Center (CNHU-HKM), Benin
2Faculty of Health Sciences, University of Abomey-Calavi, Benin
3Department of Medicine, CHUD-B/A, Benin
4University Clinic of Parasitology-Mycology, CNHU-HKM, Benin
Correspondence: Dr. Raïmi Kpossou, University Clinic of Hepato-gastroenterology, Hubert Koutoukou Maga National University Hospital Center (CNHU-HKM), Cotonou, Benin
Received: March 09, 2025 | Published: March 20, 2025
Citation: Kpossou AR,Vignon RK, Sokpon CNM, et al. Esophageal candidiasis in the digestive endoscopy unit of Cotonou’s CNHU-HKM in 2022-2023: epidemiological, diagnostic and therapeutic aspects. Gastroenterol Hepatol Open Access. 2025;16(1):42-44. DOI: 10.15406/ghoa.2025.16.00605
Introduction: Oesophageal candidiasis is one of the most common disorders of the oesophagus encountered in digestive endoscopy. The aim of this study was to describe the epidemiological, diagnostic and therapeutic aspects of oesophageal candidiasis at the CNHU-HKM in Cotonou.
Methods: This was a cross-sectional, descriptive and analytical study, with retrospective data collection from December 2022 to November 2023. The diagnosis of candidiasis was based mainly on endoscopy, and sometimes histology and/or mycology.
Results: In this study, 136 patients were identified out of 1020 admitted for upper gastrointestinal endoscopy (UGIE) at the CNHU-HKM in Cotonou, representing a frequency of 13.3%. The mean age of the patients was 48.2±18.0 years. The sex ratio was 1.3. Information on HIV status was available for 53 patients, of whom 35 (66%) were HIV-positive. Among the clinical indications for UGIE, abdominal pain was the most frequent (38.2%), followed by dyspepsia (16.2%). Dysphagia was frequently found in only 8.1% of patients. Direct examination was positive in 100% of cases (n=20). Candida albicans was the only species found in samples examined by mycology with culture. All patients had received treatment with fluconazole, with an average duration of treatment of 12.1 days +/- 2.7 days. The outcome was favourable in 75% of patients for whom information was available.
Conclusion: Oesophageal candidiasis is a relatively common condition in patients referred for OGDE. It is infrequently revealed by dysphagia and confirmed by mycological examination when suspected at endoscopy. Treatment with fluconazole is effective.
Keywords: oesophageal candidiasis, dysphagia, upper gastrointestinal endoscopy, Candida albicans, fluconazole
The oesophagus is a musculomembranous duct that transports food from the hypopharynx to the cardia of the stomach.1 It is one of the organs of the upper gastrointestinal tract (oesophagus, stomach, duodenum) that can be directly explored using upper gastrintestinal endoscopy.2 Oesophageal pathology accounts for 55.9% of upper digestive tract pathologies detected by digestive endoscopy according to a study carried out in 2013 by Camengo et al. at the Hôpital Universitaire de l'Amitié in Bangui.3 Oesophageal candidiasis (EC) is one of the most common oesophageal diseases. Infection with the human immunodeficiency virus (HIV) remains the main factor predisposing to the development of EC.4 Diabetes mellitus, immunosuppressive therapy, altered microbial flora and functional or structural abnormalities of the oesophagus have also been identified as predisposing factors for EC.5 The prevalence of EC tends to increase in HIV-uninfected patients and decrease in HIV-infected patients.3 In addition, strains of Candida albicans with reduced sensitivity to azoles have appeared. Other non-albicans yeasts such as C. tropicalis, C. krusei, C. parapsilosis and C. glabrata are increasingly reported to cause oesophageal candidiasis.6,7 Certain non-albicans species are characterised by their low sensitivity to the usual antifungal agents.2 It is therefore necessary to study the epidemiology of oesophageal candidiasis in our context. The drug most commonly used to treat oesophageal candidiasis is fluconazole (an antifungal agent).8 Nowadays, resistance to fluconazole is increasingly being described.9 Candida albicans remains the yeast implicated in the occurrence of these infections.5 However, the epidemiology of Candida infections has changed. There has been a change in the distribution of species among these fungal infections.8 To our knowledge, few published data are available on oesophageal candidiasis in Benin. The aim of this study was to describe the epidemiological, diagnostic and therapeutic aspects of oesophageal candidiasis at the CNHU-HKM in Cotonou.
This was a cross-sectional, descriptive study with retrospective data collection from 1 December 2022 to 30 November 2023. It took place in Cotonou in the digestive endoscopy department of the Centre National Hospitalier et Universitaire Hubert Koutoukou Maga (CNHU-HKM). This is the largest digestive endoscopy unit in Cotonou. The study involved patients referred for upper GI endoscopy to the hepato-gastroenterology clinic at the CNHU-HKM in Cotonou. Patients referred for upper GI endoscopy during the study period who were diagnosed with probable oesophageal candidiasis on the basis of endoscopic findings in the oesophagus were included in the study.
The diagnosis of probable oesophageal candidiasis on UGIE was based on the presence of white plaques or exudates adhering to the oesophageal mucosa.
In patients who were able to perform histopathological and mycological examination of oesophageal biopsies, these data were taken into account. The dependent variable was oesophageal candidiasis considered probable when diagnosed by oesophageal endoscopy alone, and confirmed when confirmed by histopathological or mycological examination of oesophageal biopsies. The independent variables studied were sociodemographic, clinical, aetiological, endoscopic, histological, mycological and therapeutic data.
The data was collected using a standardised survey form digitised using the KoboCollect application. Data was collected from upper GI endoscopy reports, hospital registers and consultation records. Where necessary, a telephone call to patients followed by their oral consent was used to fill in missing data. The data collected was exported from the KoboCollect application to Excel version 2019 2019 and analysed using R software. Microsoft Word 2019 was used for data entry and Excel 2016 for data organisation in the form of tables and graphs. From a regulatory and ethical point of view, authorisation was obtained from the director of the CNHU-HKM and the head of department before data collection began. Confidentiality and anonymity were respected in data storage and analysis.
During the study period, 1020 patient files were surveyed in the digestive endoscopy unit at the CNHU-HKM in Cotonou. Among these 1020 patients, we noted 136 cases of probable oesophageal candidiasis, i.e. a prevalence of 13.3%. The mean age of patients with EC was 48.2±18.0 with extremes of 7 and 90 years. The most common age group was between 40 and 59 (41.9%). The sample was predominantly male (57.3%), with 78 men and 58 women, giving a sex ratio of 1.3. Many of the patients surveyed were civil servants (44.8%). University-educated patients were the most represented in the study population (41.9%).
Diagnostic aspects
With regard to the history of patients with EC, 17 were diabetic (12.5%) and 35 were HIV positive (25.7%) (Table 1). The most frequent clinical indications for UGIE were abdominal pain (38.2%) followed by dyspepsia (16.2%) (Figure 1). Dysphagia was also relatively uncommon in 8.1% of cases.
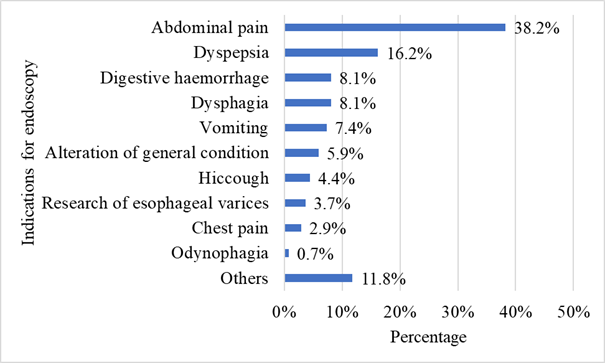
Figure 1 Distribution according to indication for oeso-gastroduodenal endoscopy among patients with oesophageal candidiasis in the digestive endoscopy unit of the CNHU-HKM in Cotonou in 2022-2023 (n=136).
|
Frequency |
Percentage (%) |
|
|
HIV |
35 |
25.7 |
|
Diabetes |
17 |
12.5 |
|
Viral hepatitis B |
7 |
5.1 |
|
High blood pressure |
6 |
4.4 |
|
Other |
4 |
2.9 |
|
No previous history |
67 |
44.8 |
Table 1 distribution according to history of patients with oesophageal candidiasis in the digestive endoscopy unit of the CNHU-HKM in Cotonou in 2022-2023 (n=136)
Other: asthma, stroke, sinusitis, pulmonary tuberculosis
Pathological examination of oesophageal biopsies was carried out in 12 patients, of whom only 7 (58%) had histopathological confirmation of oesophageal candidiasis. The remaining 5 (42%) showed no evidence of oesophageal candidiasis or eosinophilic oesophagitis. Of 21 oesophageal mucosal biopsies analysed for mycology, direct examination was positive in 100% of cases. However, 20 biopsies yielded a positive culture (95.2% frequency). Candida albicans was the only species found and no resistance to the usual antifungal agents was reported in any of the samples (Table 2).
|
Frequency |
Percentage (%) |
|
|
Pathological anatomy |
||
|
Positive |
7 |
5.2 |
|
Negative |
5 |
3.7 |
|
Not performed |
124 |
91.1 |
|
Mycology |
||
|
Positive |
20 |
14.7 |
|
Negative |
1 |
0.7 |
|
Not performed |
115 |
84.6 |
Table 2 distribution according to anatomy pathology results and mycology in patients with oesophageal candidiasis in the digestive endoscopy unit of the CNHU-HKM in Cotonou in 2022-2023 (n=136)
Therapeutic and developmental aspects
All patients received fluconazole-based treatment during the study period. The mean duration of treatment was 12.06 days +/- 2.68 with extremes of 7 and 15 days. During the study, 102 patients (75%) reported a clear clinical improvement after starting fluconazole. The evolution of the other patients remains unknown due to lack of information (not available in the files and inability to contact patients). No complications were reported in any of the patients in the study.
The mean age of the patients in our study was 48.2±18, with extremes of 7 and 90 years. The most common age group was between 40 and 59 (41.9%). The sample was predominantly male (57.3%), with 78 men and 58 women, giving a sex ratio of 1.3. These values are close to those of other authors such as Maïga et al.;10 Ouattara et al.;11 Redah et al.12 This male predominance may be explained by the fact that, according to Alsomali et al.,13 men tend to have more infections than women. The age of our patients is in line with the literature, which states that mycoses can be seen at any age. In the study by Kabore et al.,14 the average age was between 20 and 66 years, which corresponds to a period of intense sexual activity for most individuals, who may thus encounter HIV, whose infection represents a particular circumstance favouring the occurrence of oesophageal candidiasis.
With regard to acquired immunodeficiency syndrome, 35 HIV-positive patients (25.7%) among patients with EC. In more than half of cases, HIV infection is accompanied by digestive manifestations secondary to opportunistic infections, including mycoses. Yakoob et al.15 reported that oesophageal candidiasis affects 14% of HIV-infected subjects. The high number of cases of oesophageal candidiasis is partly explained by the frequent colonisation of the oesophageal segment by Candida strains from the underlying ENT sphere. Although oesophageal candidiasis is a common opportunistic infection in patients infected with the human immunodeficiency virus (HIV), the vast majority of patients in our series (61%) had a negative serological status. This means that oesophageal candidiasis is not always linked to HIV.
The rate of mycological confirmation (positive cultures) in our study was 95.2%. Our results corroborate those of several other studies where the confirmation rate was 95% according to Bonacini et al.16 Maïga et al.10 in Mali found a confirmation rate of 90%; Redah et al. 12 found a rate of 88% in Togo. The data in the literature on the most frequent species are not uniform. In our study, Candida albicans was the species isolated in all positive cultures. This species was the only one identified in 100% of cases with no other associated species. Our results are identical to those of Maïga et al.10 in Mali, who found only C. albicans as the only species identified. However, these results differ from those of Kliemann et al.17 in Brazil, who found, in a study of 163 patients, C. albicans in first place with 96.2% of cases, followed by C. tropicalis (2.5%), C. lusitaniae (0.6%) and C. glabrata (0.6%). However, in all the studies, we note a predominance of the C. albicans species. The predominance of Candida albicans can be explained by the expression of virulence factors. These factors are: surface adhesins (adhesion to epithelial cells), dimorphism (C. albicans can change from a yeast form to a filamentous form under the influence of particular environmental conditions) and the secretion of lytic enzymes (aspartic proteases, phospholipase and lipase also play an essential role in the adhesion phase).18
In this study, 102 patients received treatment during the study period with fluconazole (75%). The mean duration of treatment was 12.1 days +/- 2.7 with extremes of 7 and 15 days. Our results are similar to those of Mjabber et al.19 in Morocco in 2010 who found treatment with fluconazole for 7 to 10 days. The Candida species tested was sensitive to all the molecules. No complications were reported. We obtained results similar to those of Mjabber et al in Morocco in 2010 who found a good evolution in all cases.19
Our study shows that oesophageal candidiasis is a frequent condition in the digestive endoscopy department of the CNHU-HKM in Cotonou. It affects both young adult men and women, with a slight male predominance. The contribution of oesogastroduodenal endoscopy, histopathology and mycology is important in confirming the diagnosis. C. albicans is the only species found on mycology, and treatment with fluconazole is effective. These data need to be corroborated by prospective multicentre studies involving larger numbers of patients, in order to clarify the aetiological factors in particular.
None.
None.

©2025 Kpossou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.