eISSN: 2373-6372


Review Article Volume 15 Issue 6
1Department of Pediatric Radiology of the 1-st State Hospital, Belarus
2Dorot. Medical Center for rehabilitation and geriatrics, Israel
Correspondence: Michael D Levin, Department of Radiology, Dorot, Medical Center for rehabilitation and geriatrics, Netanya, Israel
Received: November 27, 2024 | Published: December 12, 2024
Citation: Levin MD. Esophageal achalasia or gastroesophageal reflux? Pediatric cases analysis. Gastroenterol Hepatol Open Access. 2024;15(6):174-184. DOI: 10.15406/ghoa.2024.15.00596
Classic idiopathic esophageal achalasia (EA) was a rare disease. In the last 30 years, its frequency has increased more than 1000 times. To find out the reason for these changes, 53 articles with EA in children under 18 years were selected from PubMed, including 29 articles with radiological diagnosis EA. Methods. All 29 radiographs were subjected to rentgenometric analysis to determine the true width of the esophagus and the length of the lower esophageal sphincter (LES). This allowed them to be compared with previously published standards and with the radiological characteristics of EA and gastroesophageal reflux disease (GERD). Results. 19 (66%) children had a typical picture of GERD. In 4 (14%) GERD was combined with secondary stenosis, in 3 (10%) cases congenital stenosis of the esophagus and/or LES was diagnosed at surgery and histologically. In one case, based on radiographic analysis, there was a typical picture of EA, and in 2 cases it was impossible to exclude EA. The conclusion of high-resolution manometry (HRM) in 4 patients about the presence of EA contradicted the wide opening of the LES. In 19 of 23 patients with GERD, the muscular layer of the LES and lower part of the esophagus was transected. In 3 cases balloon dilatation was performed. Discussion. Analysis literature indicates that recently have blurred the boundaries of classical EA and GERD. With GERD, complicated by rigid esophagitis and fibrous changes in the LES, there is difficulty in emptying the stomach with dysphagia syndrome. These cases are erroneously identified as true EA and operations are performed as if they were patients with EA. Thus, instead of treating the GERD and preserving the LES, sphincter is cut completely, resulting in a more severe form of GERD. Dissection of the muscular ring in the lower part of the esophagus in most cases leads to the formation of a pseudo-diverticulum, in which refluxant accumulates, causing additional severe damage to the esophagus. Neither high resolution manometry nor pH monitoring contributes to establishing the correct diagnosis. Conclusion. The increase in the incidence of EA is due to the diagnosis of EA syndrome in patients with GERD, and these patients are treated as if they had classic EA, which leads to severe complications.
Keywords: gastroesophageal reflux disease; classical esophageal achalasia; children; high-resolution manometry; x-ray study; syndrome of esophageal achalasia
Esophageal achalasia (EA) is still considered a rare disease, but over the past 50 years, there has been a sharp increase in the frequency of EA. For example, the frequency ЕА per year increased from 0.03 to 32.58 per 100,000 population (in one of the districts of Chicago),1 i.e. increased more than in 1000 times. As the analysis of the literature shows, this happened because of a change in the understanding of EA pathophysiology. Instead of a disease called idiopathic or classical EA with known characteristics of pathogenesis, manometry, and histology, EA has become a manometric syndrome. This study is devoted to the analysis of this transformation.
The purpose of this study is to analyze articles describing the diagnosis and treatment of EA in children that caused by an unexplained sharp increase (more than 1000 times) in cases of EA, as well as the lack of accurate diagnostic criteria.
From PubMed were found 53 articles including 29 articles with radiological diagnosis of children under 18 years with dysphagia, regurgitation, and chest pain, who were diagnosed with EA at different periods. Gender and age were found in only 19 of the 29 reported cases. By age, all children represented 2 different groups: (1) infants from 8 to 21 months (on average 13.5 months) and children over 6 years old - from 7 to 14 years old (on average 10 years). In both age groups, girls predominated: among babies 7/2: F/M, and at older ages 7/3: F/M.
Methods
A) Radiometric analysis. Radiographs of the esophagus and EGJ were assessed using radiometric analysis. Since the radiographs were taken with different projection magnifications, to obtain the true dimensions we determined the projection magnification coefficient, which is equal to the ratio of the known height of the first lumbar vertebra for children of a certain age to the size of its image on the radiograph. The true width of the esophagus and the length of the LES is equal to the multiplication of their values by the projection distortion coefficient. Table 1 recorded the true height of the first lumbar vertebra (L-1) in children of different ages.20
|
Age |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
|
L-1 |
1.3 |
1.4 |
1.4 |
1.5 |
1.5 |
1.6 |
1.7 |
1.8 |
1.8 |
1.8 |
1.9 |
2.0 |
2.1 |
2.2 |
2.2 |
Table 1 True height L-1 in children of different ages
If in the picture only thoracic vertebrae (D-10,11- red line) can be measured (Figure 1), then their size is 1-2 mm less than height L-1. The results of measurements in patients were compared with age standards from Table 2.21
|
|
Length of lower esophageal sphincter (cm) |
|||||
|
Age |
Up to 1 year |
1–3 years |
4–7 years |
8–10 years |
11–15 years |
21–65 years |
|
Limits |
0.7 – 1.0 |
1.2 – 1.5 |
1.5– 1.8 |
1.9 – 2.3 |
2.3 – 2.9 |
3.2 – 4.2 |
|
М± м |
0.86±0.03 |
1.40±0.02 |
1.72±0.07 |
2.10±0.05 |
2.45±0.11 |
3.60±0.08 |
Table 2 Normal length of the LES in different age groups
The LES cannot be measured in healthy individuals because the strong peristaltic wave of the esophagus drives the bolus into the stomach without stopping, despite abdominal compression. Age standards were measured in patients with a mild form of GERD, the symptoms of which appeared less than 6 months before the study, and they coincided with the results of manometric measurement of the length of the LES.21 In children under 8 years of age, the normal width of the esophagus ranges from 1.0-1.2 cm, and in children over 8 years old it is 1.3-1.5 cm.
Figure 1 shows EGJ radiographs of patients with mild GERD. As a result of increased pressure in the stomach, the LES has contracted and can be measured as the distance between the esophagus and the stomach that does not contain barium.
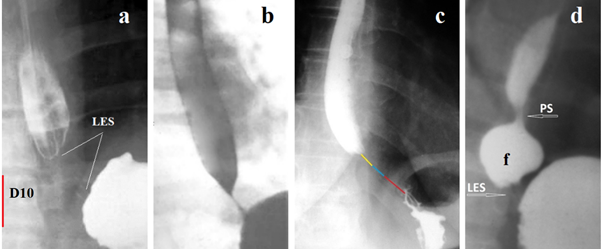
Figure 1 The X-ray image of the LES can be detected in GERD during the high pressure in the stomach, which leads to a reflex contraction of the LES. (a) The LES is visible as a zone without contrast agent between the esophagus and stomach. Knowing the height of L-1 at the age of 9 years (2.1 cm), we assume that the height of D -10 (red) is approximately 1.9 cm. Therefore, the length of the LES is 2.2 cm, i.e. equal to the age norm. (b). In the presence of inflammation, a contrast agent remains inside the LES. The cone-shaped end of the esophagus above the LES is a typical picture with GERD. (c) In a vertical position, 3 parts of the LES are shown in different colors (above the diaphragm - yellow, inside the diaphragm - blue, abdominal - red). (d) Phrenic ampulla (f) indicates a wide lumen of the esophagus. PS (proximal sphincter) is a functional sphincter that occurs with GERD. It contracts with ampullar contraction and closes the ampulla so that when it contracts, it can create high pressure between PS and LES. The LES opens at a threshold pressure and the ampulla injects a bolus
Sometimes, to assess the diagnostic accuracy of the described observation, it is not enough to analyze radiographs. In such cases, a description of clinical symptoms and the results of other research methods can help. However, the articles contain references to hypotheses that contradict scientific facts. Thus, before proceeding to cases analysis, it is necessary to get rid of false hypotheses.
А) Differential diagnosis of dysphagia in children
Progressive dysphagia of solids and liquids, regurgitation, chest pain, weight loss, and nocturnal cough, are symptoms of disorders of various diseases with impaired evacuation from the esophagus to the stomach. From an epidemiological point of view other than EA most relevant cause of pediatric esophageal dysphagia, with GERD and eosinophilic esophagitis (EoE).22 Less common are congenital and acquired stenosis of the esophagus and LES.23
(а) Heartburn: The authors describing heartburn in patients with EA acknowledge that it is still controversial whether these conditions co-exist (EA and GERD) or whether one disease transforms into the other.24 However, the authors believe that these diseases are different based on the assumptions of different authors. For example, they refer to Ponce et al, who allegedly demonstrated that patients with achalasia have lower esophageal sensitivity to acid than patients with GERD. They suggest that retrosternal burning might be due to the esophageal dysmotility of achalasia, that esophageal spasm and distention caused by achalasia might produce sensations like heartburn, that ingested irritants that remain in the aperistaltic esophagus might cause heartburn, and retained food in the flaccid esophagus can be fermented by bacteria into lactic acid. These assumptions are not scientific, as they are not supported by any scientific research. Meanwhile, since 1995 it has been known that in vitro model of lactobacillus fermentation supported the contention that true acid reflux accounted for changes in esophageal pH.25 Thus, the presence of heartburn indicates reflux of gastric acid from the stomach (GER), and excludes classic EA.
(b) Esophagoscopy: It is known that esophagoscopy without histological examination does not reveal the non-erosive form of GERD. For example, before POEM, 49 patients (53.2%) had typical GERD symptoms, as defined by a GerdQ score ≥8, while only 13 (14.1%) showed erosive esophagitis on endoscopy.26 This means that in most patients with EA syndrome was GERD. An important sign of gastroesophageal stenosis is the difficulty of passing the endoscope from the esophagus into the stomach. For example, Moody and Garret diagnosed EA in patients following lye ingestion with the typical “bird’s beak” appearance in all patients. Moreover, 7 (63%) of 11 patients had manometry with typical loss of coordination in relaxation of LES. In 5 patients esophagoscopy was conducted, which in 4 showed a dilated esophagus and difficulty in negotiating the instrument through the cardia.27 Obviously, in these cases we are not talking about a classic disease, and about EA syndrome. Saiad et al, performed a UGI endoscopic exam in 11 of 13 patients, yielding a resistance at the gastroesophageal junction in nine patients, which is not typical for true EA, and suggests stenosis in the LES.28 It is known that endoscopy only detects complications of GERD (erosions, stenoses and Barrett's esophagitis), and is not a method for diagnosing GERD. However, in all articles on EA, only endoscopy is cited as evidence that EA syndrome is not a complication of GERD. These statements are erroneous and should not be considered. Furthermore, difficulty in passing the endoscope through the EGJ indicates stenosis at the level of the LES rather than the EA.
(c) pH-monitoring: In many articles, EA was diagnosed 2-10 years after treatment failure for GERD. Currently, such cases are assessed as delayed diagnosis of EA because of misdiagnosis of GERD. However, in a significant number of patients with EA, the diagnosis of GERD was confirmed by pH monitoring. For example, Shoenut et al. у 38 (79%) of 48 patients with achalasia was acid exposure in the distal esophagus using 24-h ambulatory esophageal pH studies (total time pH < 4.0, 1.8 ± 1.9%), and in 20% (10/48), demonstrated abnormal acid exposure (total time pH < 4.0, 18.8 ± 14.8%).25 However, this picture is not complete, since pH monitoring is known to detect only severe forms of GERD. About 30% of patients with reflux remain outside of this method.2,28-30 Thus, based on pH monitoring in significant number of EA patients was GERD. Similar findings have been published both for adults31 and for children,32 including with eosinophilic esophagitis.33 However, they are ignored on the basis that they contradict the HRM, which is considered the gold standard for diagnosing EA.26 Ignoring scientific facts is contrary to the philosophy of science.
(d) High-resolution manometry: With some reservations (which will be presented below), one can agree with Shieh et al, that “HRIM is the gold standard tool for assessing esophageal function and inferring morphology”.26 However, Yeh et al. state that currently, HRM is the gold standard for an accurate diagnosis of achalasia,35 citing the article by Singendonk et al.36 Singendonk et al. believe that pressure-impedance measures may aid in the evaluation of non-obstructive dysphagia in patients by revealing abnormal motor patterns, which may explain symptom generation.36 In this study, the authors described abnormal motor patterns in patients without a specific diagnosis, which can help (but do not help) in the examination of patients, and which can explain (but do not explain) the occurrence of symptoms. In conducting clinical high-resolution esophageal pressure topography studies in patients with achalasia, Kwiatek et al. observed that after subjects sat upright between series of supine and upright test swallows, they frequently had a transient lower esophageal sphincter relaxation as well as in patients without achalasia.37 Thus, the authors who, together with the device manufacturers, began introducing HRM, confirmed that they examined patients not associated with classical achalasia, in which, as shown above, the denervated sphincter does not relax. Classic EA and EA syndrome, which is also observed with GERD, EoE, congenital and acquired stenoses, should have different names, but not manometric, but diagnostic. Because, for example, crossing the LES, useful for true achalasia, is contraindicated for GERD.
In the available literature, we found radiographic studies of 29 patients under 18 years of age who were diagnosed with EA. The results of the analysis of the described observations are presented in Table 3. The typical X-ray picture of GERD in 19 patients with EA syndrome.
|
|
GERD |
Acquired stenosis LES |
Congenital stenosis LES |
Esophageal achalasia? |
|
Number of patients |
19 |
4 |
3 |
3 |
|
After PPI treatment |
2 |
|
1 |
1 |
|
After pH monitoring |
1 |
|
|
|
|
After HRM |
4 |
|
|
2 |
|
LES crossing |
15 |
4 |
2 |
2 |
|
Ballon dilatation |
3 |
|
1 |
1- gastrostomy |
Table 3 Results of analysis of articles with radiographs of children diagnosed with EA
Figure 2 shows examples of radiographs of children with GERD whose analysis excludes the possibility of EA.
(a) A 14-year-old girl with dysphagia and vomiting. Based on radiological symptoms (dilated esophageal body, stasis of the contrast in it, a bird’s beak deformity in the EGJ) as well as on typical findings on HRM (absence EGJ relaxation), EA was diagnosed. After repeated pneumodilations of the LES dysphagia completely resolved.38
Analysis. Since the height of L-1 at this age is 2.2 cm, therefore the height of D-11 is ≈ 2 cm. What the authors called bird’s beak deformity (white arrow) is an open LES, through which the contrast agent freely fills the stomach. The length of the LES is 1 cm, which is significantly shorter than the normal LES at this age - 2.3 – 2.9 (2.45±0.11 cm). A sharp shortening of the LES indicates GERD, which is also confirmed by the presence of phrenic ampulla {a}. The esophagus is dilated just above the peptic narrowing between the two blue arrows. The diagnosis: GERD with peptic esophageal stenosis. The conclusion of HRM (absence EGJ relaxation) is erroneous, as it contradicts the obvious radiological signs: - relaxation of the LES and good esophageal emptying. The disturbance of esophageal motility was caused by peptic stenosis of the esophagus. The article does not provide information about what happened to peptic stenosis after dilatation of the LES, i.e., in another place.
(b) An 18-month-old female child was admitted with the complaints of regurgitation of feed, nonprojectile vomiting, repeated fever, and cough with occasional breathlessness for last 1 year.39 The authors diagnosed EA based on the bird’s beak appearance of the lower end of the esophagus. What the authors call “bird's beak” is an open LES with normal throughput. Analysis. The diagnosis of EA does not have a single confirmatory radiological sign, since the esophagus is not dilated, normal relaxation of the LES does not prevent the filling of the stomach. Based on erroneous interpretation of the X-ray examination, the authors diagnosed EA and balloon dilatation of the EGJ was performed. Long-term results are not shown.
(c) A 10-year-old female with Down syndrome with history of chronic daily cough and recurrent pneumonias for eight and a half years duration. Based on radiological data (diffuse dilatation of esophagus with tapering at gastroesophageal junction and small amount of contrast passed to the stomach) the authors diagnosed EA.40 Analysis. The width of the esophagus in the ampulla is 1.8 cm. Such slight dilatation of the phrenic ampulla is typical for GERD. The length of the LES from the esophagus to the air bladder of the stomach (blue arrow) is 2.1 cm, with the age norm being 1.9 – 2.3 (2.10±0.05 cm). However, the intra-abdominal part of the LES (limited by the yellow lines) is open. Only the proximal part of the LES, 1 cm long is in a contracted state. Shortening of the LES, combined with large amounts of gas and contrast material in the stomach, are also evidence of GERD. The diagnosis of EA has not been confirmed.
(d) An 8-year-old male patient. The authors diagnosed EA based on the barium examination, which revealed of an acute tapering at the gastroesophageal junction with the persistence of barium after 20 min of the swallow. Treatment by open Heller myotomy and Dor fundoplication.41 Analysis. In a horizontal position, 20 minutes after taking the contrast agent, the entire esophagus is filled with it. A width of it is 0.9 cm, i.e., significantly less than normal (1.5 cm). The length of the LES (distance between the esophagus and the stomach) is 1 cm. The age norm being 1.9 – 2.3 (2.10±0.05 cm). A significant amount of contrast agent in the stomach indicates normal evacuation from the esophagus. The narrowing of the esophagus and shortening of the LES suggests GERD with rigid esophagitis. The only sign based on which the patient was dissection of the LES, was the detection of barium in the esophagus after 20 minutes. However, on the previous radiograph, the amount of contrast material in the esophagus was significantly greater than after 20 minutes.28 This proves that after 20 minutes the esophagus was refilled with contrast material because of reflux.
(e) An 8-year-old child suffers from dysphagia and regurgitation for 2 years. Hakimi and Karimi on barium esophagogram claim LES narrowing and by arrow show bird beak sign with compensatory dilatation of the upper esophagus. Esophagomyotomy with Dor's Fundoplication was done.28 Analysis. The true height of L-1 (red line) is 1.8 cm. Therefore, the width of the lower part of the esophagus (black line) is 2.1 cm, which is slightly wider than normal (1.5 cm). The wedge-shaped continuation of the esophagus, shown by the arrow, is located at a considerable distance from the diaphragm and reaches the level of L-1. This is an opened part of the LES. Its distal part, 0.5 cm long, is in a closed state. Diagnosis: rigid reflux esophagitis with probable stenosis abdominal part of the LES.
(f) Patient ≈ 11–12-year-old.41 Hakimi and Karimi state that pre-operative contrast esophagram demonstrated achalasia and HRM demonstrated a failure of lower LES relaxation.41 Analysis. The height of D-10 is ≈ 1.7 cm. Therefore, the width of the lower part of the esophagus (black line) is 1 cm, which is significantly less than the normal width of 1.5 cm). The most distal part of the esophagus above the closed LES, 1.2 cm long (between the blue lines), is significantly narrower. It represents peptic stenosis. The length of a closed LES containing traces of a contrast agent is 1.7 cm, which is significantly shorter than the age norm - 2.3 – 2.9 (2.45±0.11cm). Narrowing rather than expansion of the esophagus, which indicates inflammation and confirms the presence of peptic stenosis, as well as shortening of the LES with traces of a contrast agent, as a sign of inflammation, allows us to conclude about GERD with reflux esophagitis, including at the LES level.
2) X-ray picture of GERD in 4 patients with peptic stenosis of the LES
In 4 cases, the diagnosis of EA was established in patients with typical clinical symptoms of dysphagia only based on X-ray examination. In 3 of them, during endoscopic examination, resistance was detected in the EGJ area, which is not typical for EA. Two of them were babies. In each of these cases, the LES was dissected (Figure 3).
(a) An example of the EGJ image in GERD. The contracted LES is visible as a thin line between the esophagus and the stomach. The presence of a contrast agent suggests mucosal inflammation of the LES. Conical end of the esophagus authors (b,c,e) call the bird's beak sign and on this basis diagnosed EA. (b) A sharply dilated esophagus and a very short distance between the esophagus and the stomach. Arrows indicate a contrast spot in the shortened LES. This is a trap of the contrast agent in the inflamed LES, which indicates deformation of the lumen. (c) The accumulation of a contrast agent in the shortened LES indicates fibrous deformation. (d) The same patient after per oral endoscopic myotomy (POEM). The uneven contours of the gaping LES indicate fibrous deformation of the walls. In another patient, after POEM (Figure 3 g), the contours of the LES are smooth.43 (e-f). Figure (e) shows the level of fluid in the upper part of the esophagus above the long esophageal narrowing (yellow arrow). The second level of fluid in the dilated esophagus is located above the closed LES with a contrast agent trap. Given the long stenosis in the esophagus, there is a high probability that it and the stenosis in the LES are caused by exposure to hydrochloric acid. The child suffered open Heller myotomy and Dor fundoplication.28
In each of the 4 cases there was esophageal dilation over a narrow and very short LES. Small volumes of contrast were trapped in the LES (red arrows). The significant shortening of the LES and signs of inflammation in the esophagus suggest acquired stenosis at the level of the LES, probably due to GERD. No radiographic evidence of EA was found. Bougienage of the LES during intensive treatment of GERD would be more justified than dissection of the LES.
3) Congenital esophageal stenosis
In 3 patients in whom EA was suspected at various stages of examination, congenital esophageal stenosis was diagnosed during surgical treatment and histological examination.
(a-b) A 9-month-old female infant intermittent vomiting, dysphagia and refusal of solid foods began after weaning. She was treated for gastroesophageal reflux. At first, radiological investigation suggested achalasia, while esophagoscopy revealed firm stenosis, which did not allow the passage of the endoscope. She underwent four endoscopic balloon dilatations that then allowed her to swallow solid food with intermittent mild dysphagia. After 17 months of esomeprazole treatment off therapy impedance-pH monitoring was normal.44 (c) After the last dilatation, EGJ patency is normal, but LES function is not visible. Since pH monitoring only detects severe forms of GERD, this child cannot be considered healthy.
(d-e) In a 1-year-old girl vomiting and progressive dysphagia began at the age of 6 months when solid food feeding was started. Esophagography revealed an abrupt narrow segment at the lower esophagus with marked proximal dilatation. The esophagoscopy findings included nonyielding lower esophageal stenosis without evidence of esophagitis. The endoscope (outer diameter, 5.8 mm) could not pass through the stenotic orifice. Resection of a narrow area with end-to-end anastomosis was performed. The diagnosis of congenital stenosis was histologically confirmed.45
(f-g). Preoperative, and postoperative barium esophagogram of a mid-esophageal congenital esophageal stenosis after successful correction.46
4) Esophageal achalasia
In three cases, based on the history, clinical data, and radiological examination, it was not possible to exclude EA. However, no manometric or histological confirmation of this diagnosis was provided.
(a) The 11-year-old girl had suffered from obstructive bronchitis for several years. She complained of problems when swallowing solid food and drank large amounts of water during her meals to support swallowing the ingested food. Insertion of the endoscope into the stomach was easily achieved. Based on the clinical symptoms and radiological picture, the authors diagnosed EA and performed a laparoscopic Heller myotomy combined with Dor fundoplication. They created the myotomy of the cardia on the lesser curvature of the stomach and distal esophagus to 5 cm above the hiatus of the diaphragm. (b,c) Contrast study of esophagus with barium sulphate obtained 2 months after surgery.47 Analysis. Sharply dilated esophagus with a cone-shaped narrowing to 3 mm for 2.5 cm at the distal end of the esophagus, which corresponds to the normal length of the LES at a given age, the absence of gas in the stomach and the free passage of the endoscope into the stomach completely correspond to the concept of EA. This is the only case (3% of 29) where the radiographic conclusion about EA is not in doubt. But to finally resolve the question of whether EA is a result of GERD, it was necessary during operations to perform a histological examination of the LES tissue.
(d) The authors report a 9-month-old female with achalasia and alacrima (also known as AAA syndrome or Allgrove or Triple A syndrome). She had gastroesophageal reflux. Despite taking histamine‐2 receptor blockers, and proton pump inhibitors, her symptoms persisted. The clinical and radiographic findings were concerning for achalasia. Endoscopy with biopsy showing mild dilation of the esophagus and a subjectively hypertensive LES. Esophageal manometry findings confirmed a diagnosis of type II achalasia. Due to the patient's feeding difficulty and aspiration risk, a gastrostomy tube was placed to ensure proper nutrition while surgical options were discussed.48 Analysis. The case report states that she had gastroesophageal reflux. “Subjectively hypertensive LES during endoscopy means difficulty in passing the endoscope through the EGJ. These data do not allow us to exclude GERD. At the same time, the length of the LES corresponds to the age norm. Evacuation from the esophagus is sharply slowed down and there is no gas in the stomach. These data, in combination with alacrima, do not allow us to exclude EA.
(е) Ever since birth, in 21-month-old female the patient did not produce tears. A vomiting started at 9 months of age. She received prokinetics, and PPIs with no improvement. A contrast esophagram series showed a bird’s beak appearance. High-resolution esophageal manometry identified type I esophageal achalasia. Alacrima was also diagnosed. The patient underwent laparoscopic Heller cardiomyotomy and partial anterior Dor fundoplication.49 Analysis. The true height of L-1 (red line) is 1.3 cm. Therefore, the length of the contracted LES (yellow line) is 0.6 cm, which is significantly less than the age norm of 1.2–1.5 (1.40 ± 0.02 cm). What the authors call the bird’s beak appearance is the open supradiaphragmatic part of the LES. A sharp shortening of the LES indicates an opening of the abdominal part of the LES, which is characteristic of GERD. A large amount of gas in the stomach and intestines indicates sufficient patency of the EGJ. A double contour of the lower part of the esophagus may be a symptom of esophagitis, which at the level of the LES worsens its patency. Conclusion. Elevated levels of GDP-mannose in AAA syndrome contribute to neuronal degradation and loss of motor skills. However, we only know for sure about an alacrima. The presence of EA is questionable.
In each of the 29 cases described, the diagnosis of EA was initially established based on an x-ray examination. At the same time, the symptoms did not have a clear description and radiometric analysis. For example, the esophagus was described as dilated when it was often either normal or smaller than normal. The main symptom defining the diagnosis of EA was “bird’s beak” appearance, “classical of achalasia cardia” or distal esophageal tapering. Under these names most often there was a contracted or/and stenotic LES. The authors never assessed its length, even though it is known to be shortened in GERD.21,50 and unchanged in EA.3,4 Almost every article in the introduction contains a reference to the degeneration of neurons in the esophageal wall in EA, but in none of the cases there was a histological examination of the dissected LES, including after open Heller myotomy. It turned out that hundreds of practitioners who described single cases or series of observations, as well as reviewers who recommended the publication of these articles, have no idea about the anatomy and physiology of the LES in normal conditions, with GERD and EA. They follow the conventional narrative, which indicates a systemic error in modern gastroenterology.
Based on clinical symptoms, it is impossible to differentiate EA from GERD, congenital and acquired stenoses of the esophagus and LES. Review by Lanzoni et al shows that pediatric dysphagia was caused by a heterogeneous group of disorders, among which in addition to EA are the most relevant EoE and GERD.22 The lack of clinical effect after PPI treatment is not evidence of EA, firstly, because PPI is not the only, or even the main method of treating GERD. Secondly, in preterm neonates, vomiting and regurgitation occurs because of immaturity of the LES and is not related to the acidity of the refluxant (see Figure 6 g). Third, in patients with GERD, when the LES is already weakened, reflux bolus with normal pH, which causes proteolysis of food proteins, can also cause inflammation in the esophagus. It is known that vomiting is a reflex expulsion of gastric contents outward because of contraction of the stomach with wide opening of the lower and upper esophageal sphincters. In patients with EA, an increase in pressure in the stomach does not change the tone of the LES, since the nervous system in it is damaged. Therefore, the presence of vomiting in children excludes true EA. Regurgitation is the expulsion of material from the pharynx, or esophagus, usually characterized by the presence of undigested food or blood. This occurs because of contraction of the overcrowded, dilated esophagus. Thus, regurgitation is impossible if the width of the esophagus is of normal size or narrow. These considerations supported the radiological evidence for the diagnosis of GERD in 19 patients where EA was excluded both as a disease and as a syndrome.
Our study showed that 19 (65%) of 29 cases had a typical radiological picture of GERD, including two patients with EoE. This was confirmed by shortening of the LES, and/or narrowing of the lumen of the esophagus, the presence in some cases of peptic stenosis in the esophagus, and a large amount of gas and contrast agent in the stomach. In 4 (13%) cases, impaired evacuation from the esophagus was caused by shortening and narrowing of the LES of a fibrous nature, which with X-ray study was often combined with spots of contrast agent at the level of the LES, and with endoscopy caused difficulty in passing the endoscope into the stomach. This is because with GERD, the mucous membrane of the LES is damaged by hydrochloric acid and pepsin, and then peptic stenosis can occur in the esophagus, as well as in the LES. Thickening of the esophageal wall in response to irritation and inflammation leads to a narrowing of its lumen and impaired peristalsis. In such cases, which correspond to the presentation of rigid esophagitis, the walls of the LES are also thick, resulting in impaired evacuation of the bolus from the esophagus into the stomach. Thus, both typical cases of GERD and acquired LES stenoses {total 23 (79%)} are the result of GERD and should be treated according to diagnosis. An example of erroneous diagnosis of EA in patients with GERD is the description of a series of 13 patients in whom X-ray examination revealed tapering at the gastroesophageal junction. Endoscopic examination was performed in 11 of 13 patients, yielding a "resistance at the gastroesophageal puckered junction in nine patients".28 The radiograph from this article shows a contrasting spot (trap) indicating fibrous narrowing in the LES (Figure 3e, f). All patients were treated with an Open Heller myotomy. At follow-up, 12 patients had complete symptom relief. "When compared to a control group presenting with GERD, they scored significantly lower in the dimensions: Foods and drinks limitations, difficulty swallowing, heartburn and vomiting" (complete symptom relief?). Myotomy of the LES in a patient with a typical picture of GERD (Figure 2.d) with a weakened (short) but functioning LES had no justification since it permanently eliminated the antireflux function of the EGJ. Only in one case (3%) in a patient aged 11 years, typical radiological and clinical signs of EA were detected.47 In two cases, for various reasons, the diagnosis of EA remained questionable.
Understanding the relationship between GERD and EA is of fundamental importance for the diagnosis and treatment of patients with dysphagia. Hallal et al described 13 patients with EA, 6 (46%) of whom were previously treated as having GERD and asthma. They concluded that achalasia symptoms may mimic common diseases in children, and therefore, may delay the diagnosis.51 In other words, they believed that the diagnosis of GERD was incorrect, which only led to a delay in the correct diagnosis of EA. At the same time, according to Nurko and Rosen, a diversity of motility disorders has been found in patients with EE including achalasia. Some evidence suggests that treatment of EE will result in some improvements in motility.34 Shieh et al. reported that before POEM, 49 (53%) of 92 adult patients had typical GERD symptoms, as defined by a GerdQ score ≥8, while only 13 (14.1%) showed erosive esophagitis on endoscopy.26 These figures about the incidence of GERD under the so-called EA are far from the truth. First, it is known that a normal endoscopy does not exclude GERD. It is used only for the diagnosis of GERD complications (erosions, stenoses and Barrett's esophagitis.52 Therefore, we have no reason to doubt that 53% of patients in whom POEM was performed were diagnosed with GERD. Secondly, pH monitoring is generally accepted for diagnosis GERD. However, as shown above, it does not diagnose GERD in more than 30% of patients. According to Shoenut et al, most untreated patients with achalasia is acid exposure in the distal esophagus using 24-h ambulatory esophageal pH studies.25 Low diagnostic accuracy pH monitoring is explained by the fact that DeMeester, without any justification, considered the possibility of physiological reflux.2 Thus, most patients who in the past, based on pH-monitoring were not diagnosed with GERD, were GERD patients, but did not receive pathogenetic treatment. Thirdly, most of the patients who demonstrated significant pretreatment reflux were asymptomatic.24 Shieh et al. showed that after POEM, 41.9% had erosive esophagitis, but only 12 had GERD symptoms. These data prove that severe esophagitis causes damage to pain-sensitive nerve elements in the esophageal wall.24 Consequently, asymptomatic patients, as well as patients who, in accordance with the Montreal definition of GERD, did not have troublesome symptoms and/or complications,53 were not examined or treated. Based on the above, we can say that almost all patients with so-called EA suffered from reflux disease. The assertion of some authors that the diagnosis of GERD in patients with EA was erroneous is refuted by numerous reports of the diagnosis of GERD using pH monitoring.25,32,33,54
Gastroesophageal reflux disease is a common clinical disease associated with upper gastrointestinal motility disorders. Each episode of reflux of hydrochloric acid, pepsin or bile causes an acute inflammatory process that increases the tone of the esophageal wall and affects esophageal pressure. It was shown that acute inflammation increases the tone of the entire digestive tract, including the stomach and anal canal. The closer the intestine to the site of inflammation, the stronger increases the tone.55 Chronic inflammation invariably induces fibrogenesis in the esophageal wall. It's happening after epithelial injury, causing proliferation and activation of resident fibroblasts. The chronic inflammation results in scar tissue formation. Thus, a chronic inflammatory process in the esophagus caused by damage to the wall by hydrochloric acid leads to the formation of an inflammatory infiltrate, which can be complicated by motility disturbances, fibrogenesis, and carcinogenesis.56
Esophageal and LES motility: An analysis of the literature indicates that during the pathogenesis of GERD, changes in pressure occur in the esophagus. With severe inflammation, infiltration, and fibrotic changes in the wall of the esophagus and especially at the level of the LES, a violation of esophageal emptying with symptoms of dysphagia may occur.
HRM: Yeh et al. state that currently, high-resolution manometry is the gold standard for an accurate diagnosis of achalasia35 and refer to the article by Singendonk et al, which states that HRM is the primary method to evaluate esophageal motility and sphincter function36 (not diagnosis EA). Shieh et al, who widely used HRM, emphasized that HRIM is the gold standard tool for assessing esophageal function and inferring morphology26 (not diagnosis EA). Different formulations indicate different understandings of the etiology and pathogenesis of EA, i.e., is EA a disease of unknown etiology with damage to the EGJ nervous system or of a manometric characteristic of the diseases, accompanied by impaired bolus evacuation from the esophagus to the stomach in GERD, EoE, in the acquired and congenital stenoses esophagus and LES. Although all authors identify the manometric diagnosis of EA with true classical EA, no one has ever studied the state of the nervous system in operated patients. In contrast, a study by Kwiatek et al showed that in patients with achalasia frequently had a LES relaxation as well as in patients without achalasia.37 This study showed that the authors who promote HRM into widespread practice, by EA do not mean classical EA at all, because with classical EA, relaxation of the LES is excluded. Although these authors avoid calling the form they invented EA diagnosis, the division of EA into 3 subtypes, on which the indication for surgical treatment depends, turns of the esophageal and LES motility into a diagnosis "esophageal achalasia”.
In our series, in 4 (21%) of 19 patients with typical radiological signs of GERD and without significant impairment in the evacuation of contrast material from the esophagus into the stomach, a diagnosis of EA was established based on HRM. This entailed the intersection of the circular muscle layer of the LES and part of the esophagus in 3 cases and dilatation of the esophagus (not the LES) in one observation. Thus, instead of treating GERD, which leads to an improvement in the function of the LES weakened by inflammation, an unjustified final elimination of LES function was carried out. The cessation or reduction in the frequency of vomiting was regarded as a positive effect, even though these patients will have to struggle with severe GERD for the rest of their lives. In connection with these data, the question arises: - Is HRM a scientifically valid, physiological, and accurate research method?
Results of GERD treatment with "EA syndrome"
The Chicago Classification, blurring the boundaries of GERD and classical EA, created a fictitious disease “EA syndrome”, which supposedly had all the signs of classical EA, but was not related to GERD. The goal of the per-oral endoscopic myotomy (POEM) or Heller myotomy operation is the vertical intersection of the circular muscle layer of the LES. However, the LES has no visible anatomical boundaries, therefore, in adults in whom the length of the LES is ≈ 4 cm, dissection is usually performed to the total length 8.3 ± 1.8 (6–15).26 In children median age 14 years, range 9 to 18 years the median length of total myotomy was 12 cm (range, 6–16 cm).43 This means that in addition to cutting the circular muscle fibers of the LES, surgeons cut the muscle fibers of the esophagus and stomach above and below the LES.
As is known, with GERD, the abdominal part of the LES opens and does not prevent reflux. However, the function of the remaining part of the LES, i.e. about 2 cm in adults, helps prevent reflux, although it periodically relaxes. Dissection of the LES leads to the following results.
А) Clinical improvement, determined by the disappearance of dysphagia. In cases where the narrowing of the lumen of the LES was due to spasm, wall thickening, or fibrotic changes, the disappearance of dysphagia can be understood, but cannot be justified surgery, because complex treatment of GERD and bougienage of the LES would lead to the to the same effect, while maintaining the LES. But the disappearance of dysphagia in 10 (30%) of 29 patients in our series with a typical X-ray picture of GERD, who had sufficient evacuation of the contrast agent into the stomach before surgery, is not clear. Why did the patient feel better after stretching the esophagus?39 Why did it get better after balloon dilatations of the EGJ if the stenosis was in the esophagus?38 This phenomenon is difficult to explain, but our studies confirm the clinical effectiveness of stretching the esophagus and LES in GERB. In infant colic, I inserted a Foley catheter into the stomach using a guidewire. The balloon of the catheter was inflated in the stomach to a diameter of 1 cm, after which it was pulled out. For most babies, colic stopped. In adults with symptoms of GERD, I gave tablets with a diameter of 1.9 to 2.3 cm to swallow, after which heartburn, chest pain and pulmonary complications immediately disappeared for a long time.60
С) According to Triggs et al., blown-out myotomy (BOM) is a postoperative complication for achalasia in 17.8% of patients after POEM.65 The diverticular-like changes occur because of dissections of the muscular layer of the esophagus above the LES. The severity of the complication is not because solid food may reside within these pockets.66 It accumulates gastric contents with low pH and pepsin, which are constantly thrown into the esophagus through the gaping LES. The figure (18%) given by these authors does not reflect the true incidence of this complication. Firstly, because the was included patients who had a post-treatment esophagram within 1 year of their follow-up manometry. Meanwhile, mucosal protrusion into the muscular window may increase over time. Secondly, the authors arbitrarily classified only those cases where the width-mouthed outpouching was >50% increase in esophageal diameter in the myotomy as BOM. In one of 29 cases of GERD on an esophagogram performed immediately after surgery, the width of the esophagus above the LES (BOM) was 47% larger of the higher located esophagus. Thus, BOM is a serious complication of myotomy, which has begun to be identified recently. Its frequency, if we neglect the artificial limitations described above, is significantly more than 18%, and obviously depends on the length of the dissection of the esophageal wall. This is another circumstance that should make the surgeon think about the need for myotomy in EA syndrome.
The increase in the frequency of EA by more than 1000 times is due to the erroneous diagnosis of EA in patients with GERD. All patients with GERD have impaired motor function of the esophagus and LES. During ontogenesis, the esophagus expands or narrows, and evacuation through the LES is disrupted. Inflammation leads to increased tone of the LES with the gradual development of fibrotic changes, which can lead to dysphagia. HRM determines changes in esophageal motor function. However, this study was designed with serious methodological flaws. As a result, the HRM parameters are not scientifically based, i.e., not reliable. In parallel with the blurring of the boundaries of GERD and classical EA, manometric examination has become a diagnosis. Instead of pathogenetic treatment of GERD and preservation of the LES, surgeons began to destroy the LES, as if they had classic EA. In all patients develop severe GERD after destruction of the LES and many patients have esophageal pseudodiverticulum. Destruction of the LES in patients with GERD complicated by EA syndrome worsens the course of GERD.

©2024 Levin. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.