eISSN: 2373-6372


Research Article Volume 16 Issue 1
PS Biopolymer GmbH & Co. KG, R&D Polymer Synthesis & Modifications, Germany
Correspondence: Matthias Knarr, PS Biopolymer GmbH & Co. KG, R&D Polymer Synthesis & Modifications, 29699 Walsrode, Germany
Received: March 20, 2025 | Published: March 31, 2025
Citation: Knarr M. Advanced analysis of alginate for treatment of gastroesophageal reflux disease. Gastroenterol Hepatol Open Access. 2025;16(1):46-49. DOI: 10.15406/ghoa.2025.16.00606
Alginates are used as the active pharmaceutical ingredients (API) in anti-reflux formulations for many years for the treatment of the symptoms of gastroesophageal reflux disease (GERD). After the consumption of such formulation a swollen gel layer (raft) is formed in the stomach of the patient. This raft is further floated to the upper part of the stomach, where its traps and neutralizes the acid content of the stomach away from the gastroesophageal junction and preventing damage to the esophageal mucosa. The characterization of such raft is mainly performed according to the so-called raft strength, where a L-hook is moved through the formed raft mass and the corresponding force is recorded. The drawbacks of this method are discussed in this contribution, which lead to high standard deviations in the results. Alternatively, a new method for the characterization of the raft is introduced, which is based on a forward extrusion process. This new method showed improved reproducibility and decreased standard deviations. As further shown in this article. the performance of the anti-reflux formulation is mainly influenced by the chemical structure of the alginate, which are used in these formulations. Alginates are a copolymer build up from 1,4-α-L-guluronic (G) and 1,4-β-D-mannuronic (M) units. Based on the modelling of this G / M ratios to the raft performance attributes, new structure property relationships could be achieved, which allow a deeper understanding of the functional related characteristics (FRC).
GERD (gastroesophageal reflux disease/acid reflux disease) is a digestive disorder, which is caused from the continuous exposure of the esophagus to acidic reflux from the stomach. The reflux of gastric acid leads to irritation, inflammation of esophageal linings, and many further complications. Treatment for GERD is generally targeted for gastric acid neutralization and suppression of gastric acid production and / or secretion. However, another key approach to alleviate the condition is to minimize the exposure of esophagus to acidic refluxate. Raft-forming anti-reflux formulations are one approach for such a treatment. When such formulations are getting in contact with the acid media in the stomach, a swollen gel layer is formed, is floating up to the upper part of the stomach and acts there as physical barrier to prevent the reflux of the stomach content back into the esophagus. As the swollen gel barrier floats over the stomach contents like a raft, these formulations are referred to “Raft-forming anti-reflux formulations”. For such treatments the hydrocolloid within the formulation acts as an active pharmaceutical ingredient (API), if the gel formation capacity is sufficient. Most of such anti-reflux formulations are using alginates as such API.1-8 Alginates are derived from brown seaweed and belongs to the family of linear co-polymers containing 1,4-β-D-mannuronic (M) and 1,4-α-L-guluronic (G) acids units. These structural elements are shown in Figure 1 and based on the acid groups in the polysaccharide backbone these products are mainly commercially available as sodium salts. The performance of these hydrocolloids is strongly dependent on the G- and M- content as well as the order of those structural elements in the polymer backbone. These architectures in the polymer chains can substantially vary between alginates from different seaweeds and / or harvested from locations around the globe.9-11
Beside of the alginate, carbonate salts are the additional key component in the anti-reflux formulation. Often sodium bicarbonate (NaHCO3) and calcium carbonate (CaCO3) are used. The calcium carbonate is mainly insoluble at neutral pH, but in the acidic environment of the stomach the CaCO3 is solubilized and the released Ca2+ ions is crosslinking the alginate, and the swollen gel layer is formed. Furthermore, the alginates are also protonated in the acid media, which promotes the swollen state.
Additionally, CO2 is released from the carbonate sources in the formulation, which supports the floating of the formed swollen gel mass and creating the needed buoyancy for the raft to float. Based on this, a physical barrier is formed, which traps and is neutralising the acid contents away from the gastroesophageal junction and preventing damage to the esophageal mucosa.6,8 Patients suffering with GERD are often also suffering with ‘acid pockets’ sitting next to the esophageal junction following intake of food and the strong alginate rafts can cap the top reducing, neutralising and / or preventing the acid reflux postprandially.4,12,13
For a high efficiency of the anti-reflux formulation, the resistance of the formed raft against shear, which is applied due to the contraction of the stomach, is of highest importance for the in-vivo performance. This article compares two characterization techniques for different anti-reflux formulation, in order to provide pre-scan tests methods which could predict the in-vivo performance.
Commercial anti-reflux formulations from Europe were evaluated. Additionally, liquid suspensions were prepared by dispersing sodium alginate in deionized water. The sodium alginate solution was mixed with neutralized Carbomer (Carbopol 974 provided by IMCD UK) solution along with preservative, sweetener and flavour to prepare model formulations based on alginate from different sources. The raft properties of this suspension were investigated after the addition of 10 and 20 ml of the formulation to 0.1 M HCl (stored at 37 °C) according to the British Pharmacopeia with the L-hook method (left part of Figure 2). Therefore, a Texture Analyser from Stable Micro System, UK is used to record the force, which is needed to move the L-hook through the formed raft layer. The maximum of the investigated force is given as the raft strength of the formulation.
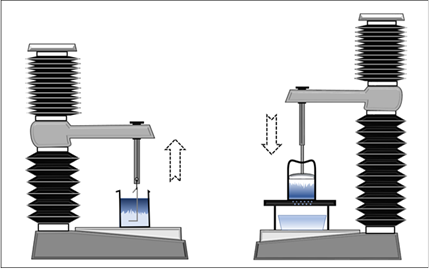
Figure 2 Raft strength acc. British Pharmacopoeia (left) vs. new Forward Extrusion Raft Gel Strength Method (right).
In order to gain further information about the resistance of the alginate raft against an applied shear, a new method has been applied. This method is based on a forward extrusion process of the formed gel mass, which was first described in 2005.14 This method is illustrated in the right part of Figure 2. The commercially available Forward Extrusion Rig HDP/FE setup from Stable Micro Systems includes an extrusion tube with a bottom plate with only one orifice in the middle. To prolong the pathway of the extrusion process this geometry was modified based on a reduction of the diameter of the extrusion tube and the bottom plate was exchanged to a honeycomb structure. Furthermore, a piston fitting ideal to the inner diameter of the cell is completing this geometry setup. These different geometry setups are shown in Figure 3.
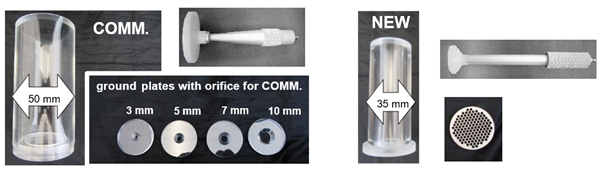
Figure 3 Commercially (COMM.) available forward extrusion Rig HDP/FE (left) vs. the new constructed one, with has a smaller diameter as well as another ground plate .
For the characterization of the raft, 10 ml of the anti-reflux formulation were given into the extrusion tube, which stands in 0.1M HCl solution in a beaker. After keeping the beaker at 37 °C for 30 mins, the HCl solution is decanted from the geometry through the wholes of the bottom plate so that the raft remains in the extrusion tube. Afterwards the geometry setup is transferred to the Texture Analyzer and the raft is extruded with the piston. The results are reported as the work of extrusion, which represents the area of investigated force multiplied by the extrusion distance.
The chemical structural composition of the alginates was evaluated via Nuclear Magnetic Resonance (NMR) spectroscopy according to ASTM F2259 – 10.15 For the anti-reflux formulation containing the alginate, the formed swollen gel layer was separated and washed to neutral pH. The Ca2+ ions, which cross-linked the alginate were removed by washing the raft with a solution of TTHA (Triethylenetetramine-hexaacetic acid). This complexation agent has a strong bonding strength to the Ca2+ ions and is therefore able to remove the calcium ions from the alginate swollen gel layer. Afterwards the alginates solutions are freeze dried and as well analysed according to ASTM F2259 – 10.
Four commercial anti-reflux formulation were purchased from pharmacies in Europe. All contains the same amount of alginate (500 mg), sodium bicarbonate (267 mg) and calcium carbonate (160 mg) with respect to 10 ml of the products. According to the description, these solutions should be used in a dosage level of 10 or 20 ml. At the max. dosage level of 20 ml only a small differentiation of the formulation could be achieved whereas at the minimum dosage level of 10 ml the differences in the performance are more visible. These data of the raft strength (L-hook) and work of extrusion for a dosage level of 10 ml are shown in Figure 4.
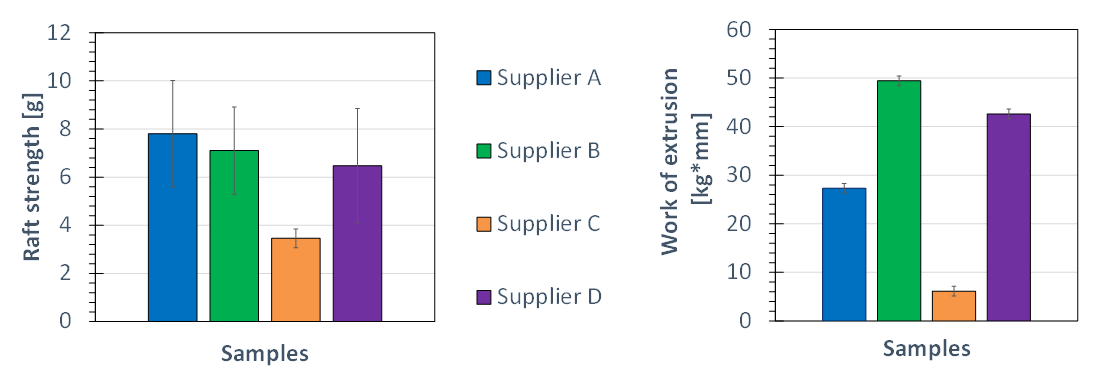
Figure 4 In-vitro performance of four commercial anti-reflux formulations containing different alginates
at the minimum dosage level of 10 ml.
The raft strength according to the data showed similar performance attributes for the anti-reflux formulation from supplier A + B and D, only the product from supplier C showed significant lower values compared to the three others. The disadvantage of the raft strength method is getting visible by the observation of the given standard deviation, which is calculated from seven measurement for each formulation. The reason for the high standard deviation of this method could be seen based on the images obtained from the formed raft in the beaker prior to the measurement. As shown in Figure 5 the raft is not homogenously formed on the upper surface of the 0.1 M HCl solution in the beaker. During the investigations the L-hook is lifted upwards through the swollen gel layer and depending on the point, where the probe is getting in contact with the raft mass, different values are obtained. Based on the structure of the formed swollen layer and the flexibility of the L-hook attached to the Texture Analyser, different values are obtained when the probe is lifted through the swollen gel mass. This explains the high standard deviations of the given method in the British Pharmacopia.
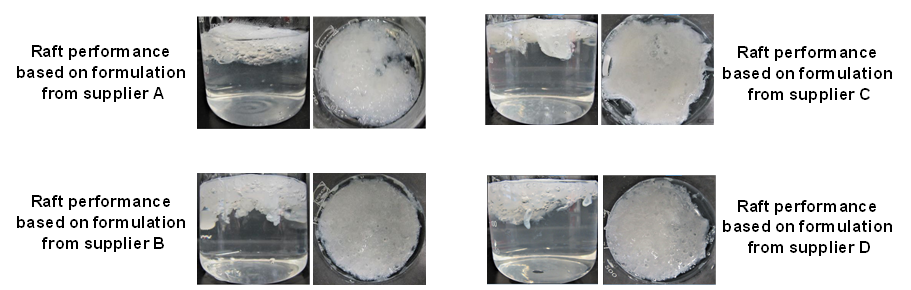
Figure 5 In-vitro performance of for different anti-reflux formulations containing different alginates
at the minimum dosage level of 10 ml.
The results of the alternative approach according to the use of the new forward extrusion cell are shown in right part of Figure 4. Here the raft is formed in the extrusion tube itself in a 250 ml beaker filled with the 0.1 M HCL and after the storage at 37 °C the acid is decanted out of the extrusion tube through the opening in the bottom plate, so that only the raft mass remains in the extrusion tube. This raft is than extruded via a piston out of the geometry. The obtained work of extrusion showed significant reduced standard deviation compared to the raft strength values. Additionally, a stronger differentiation between the four anti-reflux formulations is detected.
In a next step the alginate were isolated from the raft and analysed via nuclear magnetic resonance (NMR) technique. The relationship between the chemical structural elements of the 1,4-α-L-guluronic (G) to 1,4-β-D-mannuronic (M) content of the alginate according to the G / M ratio as a function of the raft strength as well as the work of extrusion is shown in Figure 6. Whereas the raft strength showed no sufficient correlation to the chemical structure elements of the alginate, the work of extrusion fulfilled such a relationship with a regression coefficient of nearly 99 %. Based on this data a clear trend of increasing raft gel layer resistance with increasing G / M ratio of the alginate was observed.
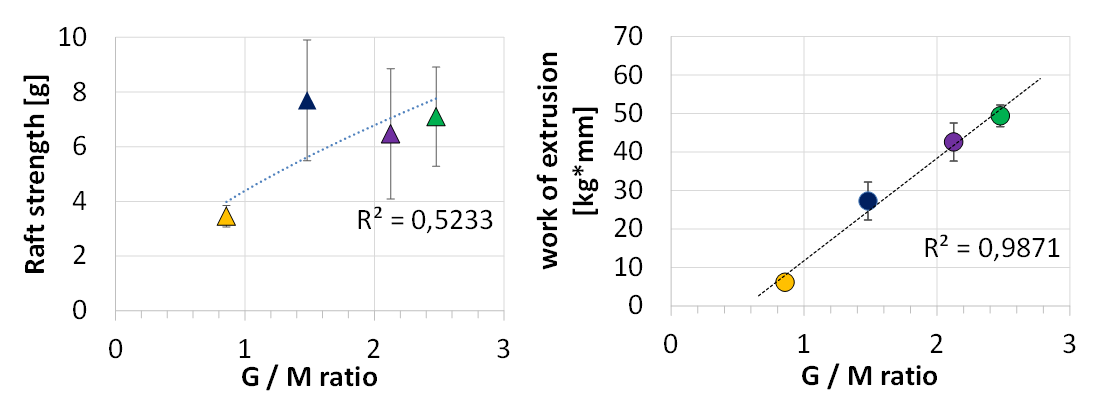
Figure 6 Correlation of the structural elements (G / M ratio) of the alginates isolated from the four different anti-reflux formulations with the in-vitro performance.
Additionally, four further anti-reflux formulation were prepared in-house based on different alginate obtained from different sources. Protanal® LFR 5/60 was used as benchmark material. All four alginates had similar viscosities in the range of 400 to 700 mPa•s at a concentration c = 10 % in aqueous solution and 20 °C. The amounts of alginate as well as the carbonate salts were kept constant similar to the formulations purchased from the pharmacies so that 10 ml of the formulation contained 500 mg alginate, 267 mg of sodium bicarbonate and 160 mg of calcium carbonate. The performance of these formulation according to the raft strength and work of extrusion is shown in Figure 7.
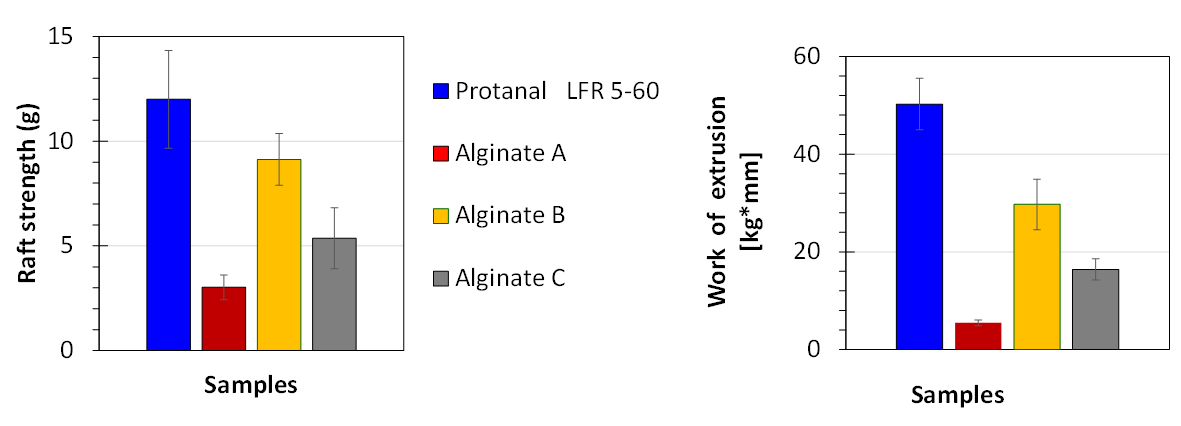
Figure 7 In-vitro performance of four anti-reflux formulations prepared with different alginates
at a dosage level of 10 ml.
In contrast to the commercial anti-reflux formulation the overall trend between the raft strength (L-hook) and the work of extrusion showed similar ranking orders. The highest values were obtained for the formulations, which contained the Protanal® LFR 5/60, followed by Alginate B and C whereas the alginate A showed the lowest raft strength performance. These data were further compared to the chemical structural elements of the alginates (G / M ratio). These data are given in Figure 8.
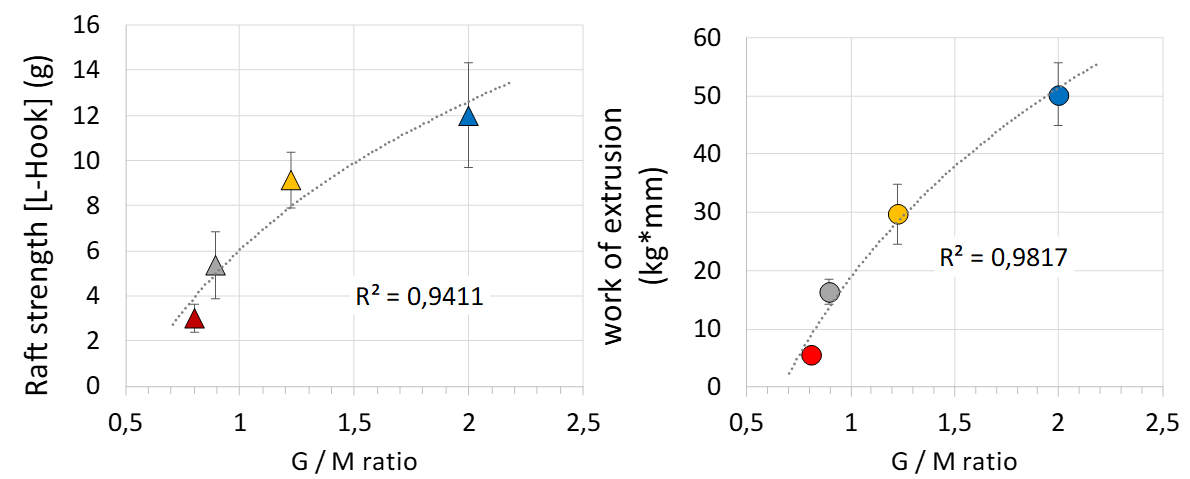
Figure 8 Correlation of the structural elements (G / M ratio) of the alginates isolated from the four different anti-reflux formulations with the in-vitro performance.
For these in-house prepared formulations, the L-hook raft strength as well as the work of extrusion showed increasing performance attributes with increasing G / M ratio. The raft strength (L-hook) showed a correlation coefficient of 94% to the G / M ratio, whereas the work of extrusion could be fitted to the G / M ratio with a regression coefficient of 98 %. These results underline the insights, which the new work of extrusion investigation technique is able to provide with respect to the in-vitro performance of the anti-reflux formulation.
Alginates are used as the active pharmaceutical ingredients (API) in anti-reflux formulations for many years for the treatment of the symptoms of gastroesophageal reflux disease (GERD). This treatment is mainly based on the formation of a raft swollen layer under the acid conditions in the stomach. As shown in this article the resistance of this swollen layer raft is mainly driven by the G / M ratio of the alginates. Alginates are a copolymer build up from 1,4-α-L-guluronic (G) and 1,4-β-D-mannuronic (M) units in the polysaccharide backbone and the G / M ratio differ based on seaweed species and the region where the alginate was harvested. Protanal® LRF 5/60, with high
G / M ratio, was found to be the best performing alginate to achieve a robust raft performance. The characterization of such rafts is mainly performed according to the so-called raft strength, where a L-hook is moved through the formed raft mass. The drawback of this method are the high standard deviations, which are based on the non-homogenous structure of the rafts. A new method for the characterization of the raft has been introduced, which is based on a forward extrusion process. This new method showed improved reproducibility and decreased standard deviations. Based on this method and the knowledge about the chemical structural elements in the alginate polymer chain, new structure property relationships could be achieved, which allow a deeper understanding of the functional related characteristics (FRC).
The author is gratefully acknowledging the support and the contributions of Kerstin Schmidt, the analytical lab team as well as the colleagues from the workshop in Bomlitz, Germany for the construction of the various forward extrusion cell prototypes.
None.

©2025 Knarr. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.