eISSN: 2575-906X


Research Article Volume 8 Issue 1
1South Dakota Game, Fish and Parks, McNenny State Fish Hatchery, 19619 Trout Loop, Spearfish, SD 57783, USA
26708 East 76th Street, Tulsa, OK 74133, USA
3South Dakota Game, Fish and Parks, 523 East Capitol Avenue, Pierre, SD 57501, USA
Correspondence: Michael E Barnes, South Dakota Game, Fish and Parks, McNenny State Fish Hatchery, 19619 Trout Loop, Spearfish, SD 57783, USA
Received: March 03, 2025 | Published: March 26, 2025
Citation: Henderson RE, Wollman KM, Pasbrig CA, et al. Age, growth, and mortality of giant floater (Pyganodon grandis) in eastern South Dakota, USA natural lakes and reservoirs. Biodiversity Int J. 2025;8(1):1-8. DOI: 10.15406/bij.2025.08.00215
The freshwater mussel Giant Floater Pyganodon grandis is native to North America. This study documented Giant Floater distribution, age, growth, and mortality rates in eastern South Dakota, USA lakes and reservoirs. The trophic status of water bodies where Giant Floater were collected was also assessed. Live and dead Giant Floater shells were observed in 13 natural lakes and eight reservoirs in the Big Sioux, James, Minnesota, and Missouri River basins. No Giant Floater were collected from waters in the Red River basin. Trophic State Index levels in the water bodies containing Giant Floater ranged from 55.8 to 98.3. Mean values were nearly identical between lakes and reservoirs at 68.9 and 68.7, respectively. Giant Floater ages ranged from 4-to-11 years. Mean age was not significantly different between natural lakes and reservoirs, at 6.1 and 7.8 years, respectively (p = 0.054). Mean estimated length was not significantly different between lakes and reservoirs at 9.71 cm and 12.90 cm, respectively (p = 0.115). Similarly, mean growth coefficient (K) was not significantly different between lakes at 0.27 cm/year and reservoirs at 0.23 cm/year (p = 0.406). Mean annual mortality was 36.7% and was not significantly different between lakes and reservoirs (p = 0.054). A significant negative relationship was found between Giant Floater maximum age and natural lake trophic state (R2 = 0.394, p = 0.022), but no such relationship was observed in reservoirs. There were no significant linear relationships between growth, estimated length, instantaneous mortality, annual mortality, and the trophic state of natural lakes or reservoirs.
With nearly 900 species in six families, freshwater mussels (order Unionida) are a large component of global animal diversity.1 The Unionidae is the largest family within the Unionida, containing approximately 700 species.2,3 Many unionid mussel species have been described as threatened, vulnerable, or endangered in North America, creating a need for additional research into the life history of freshwater mussels.1,4
Giant Floater Pyganodon grandis are native to the Mississippi and Missouri River drainages in the United States.5 They are found throughout the Mississippi River basin, several Gulf drainages, and into Canada, being hosted by over 35 fish species.6-9 Giant Floater are identified by a thin, multicolored elongated shell, with a yellowish-tan-to-green periostracum in younger shells that becomes light-to-dark-brown in older mussels.7-11 Their shell has a thickened hinge, elevated beak, and a white and light pink nacre with underdeveloped teeth.7-11 Shell lengths can reach up to 23 centimeters with ages averaging at 12 years.9,12,13
Giant Floater exhibit an opportunistic life strategy with rapid growth, a short life-span, and early maturation.14,15 All these adaptations allow for rapid colonization of, and persistence in, frequently disturbed and unstable ecosystems.1,9,14,15 Giant Floater are habitat generalists, allowing them to have a much higher survival rate than most Unionid species.15
In the South Dakota, Giant Floater was the most abundant and widespread mussel species, occurring in all 14 river drainages.16 In addition to its presence in South Dakota’s rivers, it is also found in abundance in the eastern lakes and reservoirs of the state.16-18 Despite its widespread occurrence, abundance, and presence in both natural lakes and reservoirs in South Dakota, Giant Floater natural history in the state has not been previously described.
This study evaluated Giant Floater age, growth, and mortality in natural lakes and reservoirs. In South Dakota, 69% of publicly-owned lakes are either eutrophic or hypereutrophic, likely impacting mussel health and abundance of South Dakota.19 The objectives of this study were to test the hypotheses that Giant Floater growth and mortality rates would be positively related to water body tropic status, while life span would be negatively related.
Lakes and reservoirs in Eastern South Dakota were surveyed for freshwater mussels from 7 May to 9 August 2017. Water bodies were selected using a similar protocol as Pasbrig et al.16 Sample sites (n = 116) were proportionally and randomly assigned to publicly-owned waterbodies within each of the six major river drainage basins in Eastern South Dakota based on basin size. However, some publicly-owned waterbodies could only be accessed through privately-owned land. If permission from the private landowner could not be obtained to access a lake, or if water levels precluded surveying, that specific lake was randomly replaced with a different water body within the same river drainage basin.
Two person-hour timed searches were performed at each lake or reservoir survey site.22 Each search began at the nearest lake access point or the most suitable habitat, avoiding cattail-dominated shoreline. Because of high turbidity and low visibility, tactile searches using a zig-zag motion parallel to the shoreline in water up to 1.5 m deep were performed. The two-person-hour search was divided into two equal intervals to allow specimens from the two surveyors to be combined and properly recorded.23 GPS coordinates were taken at the start, middle, and end locations of the search area to calculate total length and mussel locations within the search area. All live Giant Floater and shells from recently-dead Giant Floater were collected and identified following taxonomy by the Freshwater Mussel Conservation Society (2023). (Figure 1)
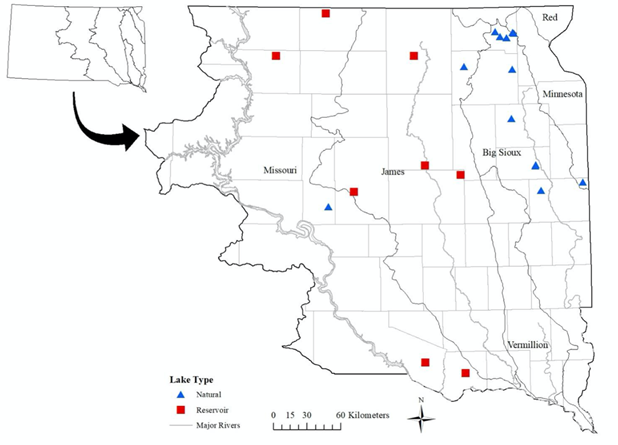
Figure 1 Locations in Eastern South Dakota natural lakes and reservoirs where Giant Floater were collected (n=21).
Water transparency (turbidity) was measured in each water. Secchi disk and Secchi tube measurements were obtained between 10:00 A.M. and 2:00 P.M. (Lind 1979). In lakes with excessive turbidity above the range of Secchi disk criteria, Secchi tube measurements were converted to Secchi disk equivalents using the equation:
Secchi Disk = 15.68 × 0.82 (Secchi Tube)
This equation was based on regression of paired Secchi depth and Secchi tube readings (R2=0.694, p < 0.01; n=42) (Figure 2). Trophic State Index (TSI) was calculated using the following formula:25
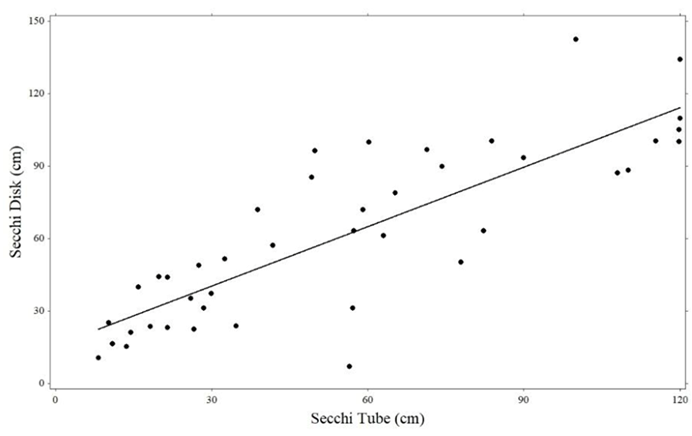
Figure 2 Linear regression relationship of paired Secchi tube and Secchi disk readings in Eastern South Dakota lakes and reservoirs. (n=42).
Trophic State Index = 60 − 14.41 ln (Secchi Disk)
Shell measurements were used to calculate Giant Floater age and growth.20,21 In those waters where Giant Floater were detected, a random sample of 10 mussels was used for age and growth measurements as described by Neves and Moyer.26 A cut from the umbo towards the ventral margin of the shell was made using a wet tile saw with a diamond-studded blade. The cut shell was epoxy-bonded to a 10.8 cm x 18.4 cm acrylic slide. A second cut was made after a minimum 24-hour drying period to create a thin 2-mm section. Fine sandpaper was used to polish the thin section. The slide was viewed with a dissecting microscope (model SZX12, Olympus Corporation of the Americas, Pennsylvania USA) using imaging software (CellSens Dimension 1.16, Olympus Corporation of the Americas, Pennsylvania USA) to measure growth increments at each age.27 True annuli were counted and measured from the umbo to the shell margin along each thin section.28
Growth of each mussel was modeled using the von Bertalanffy growth curve13,29 calculated with the formula:
𝐿𝑡 = 𝐿𝑖𝑛(1 − 𝑒−𝑘(𝑡−𝑡0))
where Lt is the length (cm) at time t (age in years), Linf is the estimated length (cm) at time infinity, and the growth coefficient (K) is a measure of how quickly the specimen will reach Linf.20 Back-calculated length-at-age for each specimen was required to calculate average length-at-age and standard deviation for each age class within each lake basin.30
The dorsal-ventral dimension of live and dead shells, coinciding with the thin sections used for aging, was used to calculate age-length keys following Ogle.31 Mean length-at-age was calculated, and 1-cm length bins were assigned to group unaged specimens to a class. Mortality rates were estimated from catch-curves obtained from growth curves and length-at-age keys with the assumptions of constant recruitment, equal survival among age classes, steady survival from year to year, natural consistent mortality each year, and fitted catch-curves that are not biased to a certain age class.30,31 Instantaneous mortality rate (Z) was calculated using catch (C) at time t (in years) with the following formula:
𝑍 = log(𝐶𝑡) − log(𝐶𝑡+1)
Instantaneous mortality (Z) was then converted to total annual mortality rate (A) using the following formula:30
𝐴 = 1 − 𝑒−𝑍
Maximum age (Amax), growth coefficient (K), estimated length (Linf), instantaneous mortality rate (Z), and annual mortality (A) data between natural lakes and reservoirs were analyzed using t-tests. If variances were unequal, Satterthwaite t-tests were used. Linear regression was used to analyze maximum age (Amax), growth coefficient (K), estimated length (Linf), instantaneous mortality rate (Z), and annual mortality (A) data in relation to the water body trophic state index. were compared between lake trophic states. The first three years of age were not included in the analysis of instantaneous mortality (Z) and total mortality (A) rates because Giant Floater do not fully mature until the age of 4 or 5.32,33
Live Giant Floater and dead Giant Floater shells were observed in 13 natural lakes and eight reservoirs in the Big Sioux, James, Minnesota, and Missouri River basins. No Giant Floater were collected from waters in the Red River basin. Trophic State Index levels in the water bodies containing Giant Floater ranged from 55.8 to 98.3, with mean values nearly identical between lakes and reservoirs at 68.9 and 68.7, respectively (Table 1).
|
Lake Name |
County |
Type |
Trophic State |
TSI |
Age and Growth |
Mortality |
|||||
|
Linf |
K |
Amax |
N |
Z |
A |
N |
|||||
|
Natural Lakes |
|
|
|
|
|
|
|
|
|
|
|
|
Pierpont |
Day |
Natural |
E |
55.8 |
8.76 |
0.45 |
7 |
10 |
0.23 |
20.4% |
91 |
|
Enemy Swim |
Day |
Natural |
E |
59.6 |
10.74 |
0.19 |
8 |
10 |
0.38 |
31.5% |
78 |
|
Six Mile |
Marshall |
Natural |
E |
59.6 |
8.39 |
0.31 |
7 |
10 |
0.32 |
27% |
97 |
|
Clear |
Marshall |
Natural |
E |
60 |
8.36 |
0.23 |
7 |
9 |
0.47 |
37.5% |
108 |
|
Mud |
Marshall |
Natural |
E |
64.7 |
11.14 |
0.15 |
5 |
10 |
0.45 |
36.4% |
82 |
|
Roy |
Marshall |
Natural |
E |
65.8 |
7.73 |
0.29 |
9 |
8 |
0.12 |
11% |
71 |
|
Florence |
Hamlin |
Natural |
H |
67.6 |
11.81 |
0.15 |
8 |
10 |
0.25 |
22% |
121 |
|
Oakwood West |
Brookings |
Natural |
H |
68.2 |
14.47 |
0.17 |
5 |
3 |
0.65 |
47.8% |
28 |
|
Dry |
Codington |
Natural |
H |
69.7 |
9.29 |
0.39 |
5 |
10 |
0.32 |
27.3% |
59 |
|
Oak |
Brookings |
Natural |
H |
71.9 |
8.29 |
0.34 |
4 |
3 |
-0.13 |
-13.5% |
167 |
|
Peno |
Hyde |
Natural |
H |
73.6 |
12.18 |
0.21 |
5 |
10 |
0.69 |
50% |
79 |
|
Greys |
Marshall |
Natural |
H |
80.6 |
6.79 |
0.28 |
5 |
10 |
0.13 |
11.8% |
80 |
|
Dry |
Hamlin |
Natural |
H |
98.7 |
8.33 |
0.31 |
4 |
6 |
0.14 |
13.4% |
28 |
|
Reservoirs |
|
|
|
|
|
|
|
|
|
|
|
|
Dakotah |
Hand |
Reservoir |
E |
59.6 |
13.85 |
0.19 |
9 |
7 |
0.37 |
30.9% |
94 |
|
Dudley |
Spink |
Reservoir |
E |
60 |
7.75 |
0.28 |
6 |
10 |
0.47 |
37.4% |
86 |
|
Straum |
Beadle |
Reservoir |
E |
62 |
16.52 |
0.15 |
6 |
10 |
1.13 |
67.7% |
229 |
|
Wagner |
Charles Mix |
Reservoir |
E |
64.4 |
10.82 |
0.34 |
9 |
10 |
0.21 |
18.6% |
105 |
|
Tyndall C.F.P. |
Bon Homme |
Reservoir |
H |
70.8 |
12.70 |
0.21 |
8 |
3 |
0.25 |
22% |
47 |
|
Hiddenwood |
Walworth |
Reservoir |
H |
74 |
22.09 |
0.09 |
6 |
9 |
1.18 |
69.1% |
140 |
|
Wolff |
McPherson |
Reservoir |
H |
78.2 |
6.90 |
0.36 |
7 |
10 |
0.12 |
11.5% |
129 |
|
Elm #1 |
Brown |
Reservoir |
H |
80.9 |
12.58 |
0.23 |
11 |
10 |
0.45 |
36.4% |
334 |
Table 1 Eastern South Dakota natural lakes and reservoirs, with trophic state and index, where three or more Giant Floater were sampled (N = 21), along with Giant Floater estimated length (cm) (Linf), growth coefficient (cm) (K), maximum age (years) (Amax), number of aged shells (N), instantaneous mortality rate (Z), annual mortality rate (A) and number of specimens (N). Trophic state: E= eutrophic and H= hypereutrophic. TSI value was calculated on a 0-100 scale
Mean Giant Floater age was 6.1 years (SD = 5.6) in natural lakes and 7.8 years (SD = 7.1) in reservoirs, with no statistically-significant difference (p = 0.054; Table 2). Ages ranged from 4-to-11 years. Mean estimated length (Linf) was 9.71 cm in natural lakes and 12.90 cm in reservoirs and was also not significantly different (p = 0.115). Similarly, the mean growth coefficient (K) was not significantly different (p = 0.406) between lakes at 0.27 cm/year and reservoirs at 0.23 cm/year. Mean annual mortality was 36.7% and was not significantly different between lakes and reservoirs (p = 0.208).
|
Age and Growth |
Mortality |
|||||||
|
Site Type |
Age |
Linf |
K |
N |
Z |
A |
N |
|
|
Natural |
6.08 |
9.71 |
0.27 |
13 |
0.31% |
24.80% |
13 |
|
|
Reservoir |
7.75 |
12.9 |
0.23 |
8 |
0.52% |
36.70% |
8 |
|
Table 2 Mean Giant Floater age, estimated length (Linf) (cm), growth coefficient (K) (cm), instantaneous mortality (Z), and annual mortality rate (A) for Eastern South Dakota natural lakes and reservoirs.
There was a significant negative relationship between Giant Floater maximum age and natural lake trophic state (R2 = 0.394, p = 0.022; Figure 3). However, no such relationship was observed in reservoirs (R2 = 0.099, p = 0.447). There were no significant linear relationships between growth, estimated length, instantaneous mortality, annual mortality, and the trophic state of natural lakes or reservoirs (Figures 4-7).
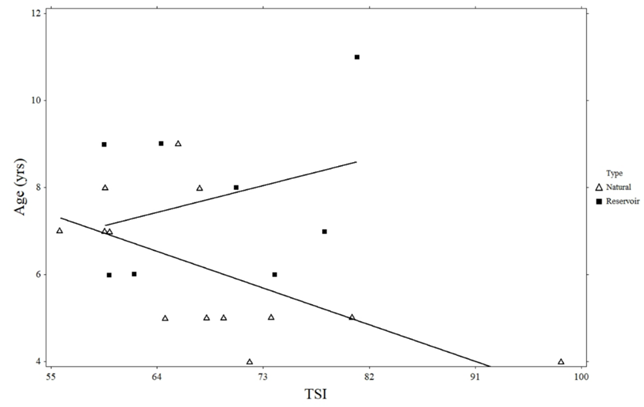
Figure 3 Linear regression of Giant Floater maximum age and trophic state index (TSI) for Eastern South Dakota natural lakes and reservoirs.
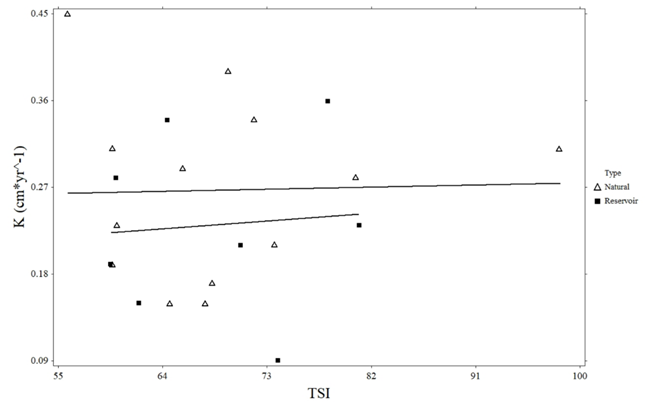
Figure 4 Linear regression of Giant Floater growth coefficient (K) and trophic state index (TSI) for Eastern South Dakota natural lakes and reservoirs.
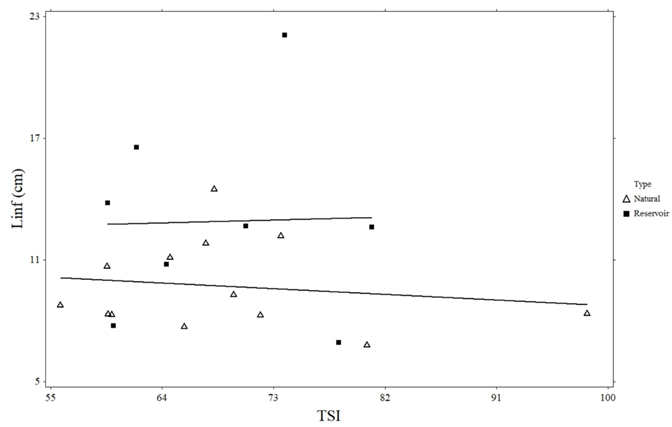
Figure 5 Linear regression of Giant Floater estimated length (Linf) and trophic state index (TSI) for Eastern South Dakota natural lakes and reservoirs.
The widespread prevalence of Giant Floater in Eastern South Dakota is not surprising. Giant Floater have been reported previously throughout South Dakota waters.16 Because of its rapid growth and early maturity, it can rapidly colonize and persist in a wide variety of habitats.14,15 Giant Floater is an opportunistic habitat generalist with much higher survival than many other unionid species.9,15 It is especially tolerant of the turbid, silty, and eutrophic conditions typical of Eastern South Dakota lakes and reservoirs.1,9,34
The ages observed in this study are similar to the maximum age of 9‑to‑12 years and age-to-maturity of 4-to-5 years reported in other geographically different populations.13,33 Although not statically-significantly different, the results of this study suggest that Giant Floater may live longer in reservoirs compared to natural lakes. In Eastern South Dakota, reservoirs are generally deeper than natural lakes. Giant Floater growth has been documented to be slower in deeper lakes compared to shallower waters12, likely because of the lower water temperatures associated with deeper water35,36 or reduced light penetration decreasing algal abundance.37 Slower growth is also positively related to longer mussel life spans.13,20,38
The negative relationship observed between Giant Floater age and the trophic level of natural lakes could have multiple causes. While nutrient levels have been documented to increase mussel growth,21,39-42 and increased growth is associated with shorter life spans,13,20,38 there was no association between trophic level and growth in either lakes or reservoirs in the current study. Rather it is more likely that the high nutrient levels associated with lake trophic level are leading to increased Giant Floater mortality and lower ages. High nutrient levels are a potential cause of freshwater mussel mortality.43 Bauer20 reported that high nitrate levels caused increased mortality in another freshwater mussel species. Most of the natural lakes in Eastern South Dakota are either eutrophic or hypereutrophic, including the natural lakes in this study,19 which could potentially be negatively impacting Giant Floater age. The impacted environmental conditions of lake and reservoirs, and the fluctuating fish populations in Eastern South Dakota, can be stressful for many mussel species, although Giant Floater appear to be relatively well-adapted to these less-than-ideal conditions.9,19 Other factors not examined in this study, such as competition from non-native zebra mussels (Dreissena polymorpha) and Asian clams (Corbicula fluminea), may also have impacted the results.44-46
In conclusion, the results of this study indicate that Giant Floater are well-adapted to the nutrient-dense lakes and reservoirs of Eastern South Dakota. Using the data collected in this study as a baseline, additional future studies can examine the potential effects of changing environmental conditions, such as increased temperatures, that can affect freshwater mussels.47,48
This study was funded in part through State Wildlife Grant T-61-R-1. Additional funding was provided by the South Dakota Department of Game, Fish and Parks, and the South Dakota Agriculture Experiment Station. Katlyn Beebout and Blake Roetman provided field sampling assistance, and Isaac Polak assisted with manuscript formatting.
None.

©2025 Henderson, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.