eISSN: 2378-315X


Review Article Volume 12 Issue 6
Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Campeche, Mexico
Correspondence: Mex-Álvarez Rafael Manuel de Jesús, Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Campeche, Mexico
Received: December 29, 2023 | Published: December 12, 2023
Citation: Mex-Álvarez RMJ, Estrada CEA, Guillen-Morales MM, et al. Residual concentration of Amikacin in expired drugs. Biom Biostat Int J. 2023;12(6):192-194. DOI: 10.15406/bbij.2023.12.00402
Antibiotics are antibacterial compounds produced by microorganisms that arose due to the need to treat infectious diseases;1,2 among antibiotics, aminoglycosides are widely used in infections against aerobic Gram-negative bacteria and can be administered together with Betalactams in the case of extrarenal infections.3,4 Aminoglycosides are characterized by the basic chemical structure of a combination of aminated (aminoglycosides) or non-aminated (glycosides) sugars linked to aminated (aminocyclitols) or non-aminated (cyclitols) cyclic alcohols, a chemical structure that gives them high solubility in water.5,6 Recent studies have demonstrated the risk posed by antibiotics as organic environmental contaminants due to their effect against pathogenic microorganisms in animals and humans, as well as their use in food preservation.7 All this has increased their production and consumption, allowing large discharges of antibiotics into water bodies with manifestations of microbial resistance in the study areas.8Therefore, the present work aims to quantify the residual concentration of amikacin in expired drugs, in order to estimate the impact that aminoglycosides can have on the environment and on the health of the population.
The expired drugs were chosen from a stock collected during a campaign to collect expired drugs organized by the Drug Information Center at the Faculty of Chemical and Biological Sciences of the Autonomous University of Campeche. The selection criteria for the samples were as follows: injectable solution, as a pharmaceutical form and the number of ampules per sample, 2 or more ampules from the same lot.
For this test, silica gel plates were used, preserved and protected from humidity. A glass chamber was used in which the bottom contained a 0.5 cm high layer of solvent system; the samples to be analyzed were applied at a distance of 2 cm from the lower edge of the plate and leaving 1 cm on each side. The application was made with the help of a Pasteur pipette modified as a capillary, applying the samples in the form of a compact spot no wider than 6 mm. The distance covered by the samples, considering the point of origin, was three quarters of the length of the plate. After the application of the sample, it was left to dry in the air and then placed in the chamber hermetically covered at room temperature until the mobile phase ascended to the desired height, when this happened the plate was removed, the solvent was allowed to evaporate and the plate was developed by impregnating it with a solution of ninhydrin at 1 percent (m/v) in a mixture of butyl alcohol and pyridine (100:1): pyridine (100:1), the excess was carefully and quickly removed and exposed to 110°C in an oven for approximately 15 minutes. The presence of pink to violet spots at the corresponding height in distance and measured from the origin was compared to that obtained with the control sample.
The different samples of expired drugs were analyzed with an Infrared spectrophotometer (TrueDefender FTX), with the help of its database, the fingerprint region of the spectrum was compared to corroborate the identity of the active ingredient of each drug.
For this assay, solutions of 100ppm concentration were prepared in a phosphate buffer of pH 7.4, from which 5mL aliquots were taken and 1.5mL of the 1.25% (m/v) ninhydrin reagent was added. Subsequently, they were heated in a water bath at 95°C for 15 minutes, after which time they were placed in an ice water bath to be read in the spectrophotometer. The absorbance was evaluated in samples at room temperature at a wavelength of 400nm. This test was performed in triplicate for each sample and the calibration curve was obtained using glycine as the standard solution.
This is particularly important in the case of antibiotics because, in addition to the classic problems attributed to drugs in general, when they contaminate water, soil or air they can generate resistance in exposed microorganisms.7,9,10 Aminoglycosides are a special group of antibiotics because they are highly soluble in water, which increases their potential to contaminate water bodies,11 so it was decided to study them; in this research work, according to the selection criteria, a total of 13 samples of amikacin with up to 60 months of expired expiration were obtained, and samples of non-expired antibiotic were also acquired as a control in the experiments. The characterization and identity of the samples were performed by chemical methods, the structure of amikacin was corroborated by infrared spectroscopy and thin layer chromatography. The identity of the active principle of the drugs (amikacin) was verified by IR spectroscopy (Figure 1).
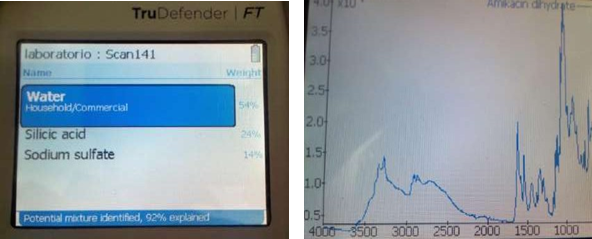
Figure 1 Photographs of the IR study: left, image of the screen of the IR equipment used for the determination; right, image of the spectrum of Amikacin obtained.
The IR spectrum is classically divided into two parts: in the functional group region where it is possible to observe which groups are present or absent in the chemical structure; the second part is the fingerprint region that allows comparing the spectrum of an unknown compound with a library to elucidate what type of compound it is. The IR spectrum highlights the presence of a double shoulder-type peak of low intensity between 3000 and 3500 (approximately at 3300) cm-1 characteristic of the amino groups; the spectra obtained were contrasted with the spectra of the control drug and with the database (library) of the equipment, proving that the drugs contained the established active principle.
Likewise, chromatoplates were performed as identity tests, which are the tests established by pharmacopoeias; various systems were evaluated in order to find the best solvent system that would serve for the resolution of the mixture (better differentiation between the two active principles). Of the various systems tested, the most suitable for the characterization of Amikacin, the Formol/isopropanol/citric acid [2:1:2] system was finally selected because it showed the best resolution.
Chromatoplate with a Nihydrin solution and heating in oven at 105ºC to find the amino compounds. Amikacin is a very polar compound and therefore very soluble in water and insoluble in organic solvents, even very polar ones such as methane or ethanol; this influences the solvents used as mobile phase for chromatography: chloroform, ethyl acetate, methylene chloride, acetone; they were not good systems. Formaldehyde greatly improved TLC resolution due to the interaction of the carbonyl group of the solvent with the functional groups of the aminoglycoside.
In addition to the usefulness of formalin as a solvent for resolving the aminoglycoside mixture, as demonstrated when pure solvents were run, the literature mentioned that very polar compounds such as amines and alkaloids separate better in both basic and acidic aqueous media; for this reason basic aqueous (with NaOH, KOH and NH4OH) and acidic inorganic [HCl and B(OH)3] and organic (acetic, tartaric and citric) solutions were also tested. Amines ionize in acidic media to form ammonium salts and in basic media they acquire their mainly non-ionized form. As the aminoglycosides have very similar polarity, the separation of the active principles is complicated; the acidic medium was the ideal medium for separating the active principles (Figure 2). Among the alcohols tested (EtOH, MeOH, isopropanol, propanol, glycerol) the best was isopropanol.
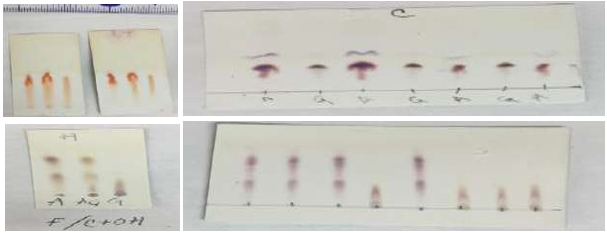
Figure 2 Photographs of TLC chromatography of aminoglycosides: top left, amikacin co-chromatography; top right separation of expired drug samples [both plates above were separated in formalin/isopropanol/citric acid 2:1:2]. Bottom left, amikacin co-chromatography; top right separation of expired drug samples [both plates below were separated with Formaldehyde/EtOH/Tartaric acid 2:1:2].
Once the presence of the active principle was identified and verified, it was quantified by UV-Vis spectrophotometry using the ninhydrin method, first a standard curve was established with glycine and then two calibration curves were made using the control drug. The ninhydrin method is based on the formation of a purple colored compound that is proportional to the concentration of the amines, the difficulty of the method is the heating; heat is necessary for the reaction to take place so that if it is not heated enough but causes the evaporation of water from the reaction mixture which concentrates it and alters the concentration of the chromogen, therefore special care must be taken in performing the technique (covering the test tubes to avoid loss of water). As the colored compound is formed by the coupling of the amino group with ninhydrin it is more specific than the ultraviolet method and is easier to perform in the laboratory.12,13 Colorimetry uses a spectrophotometer and plastic or glass cells which is more economical and accessible to most laboratories.
In this work, a calibration curve was obtained with a fairly acceptable linear correlation coefficient for the ninhydrin method (R=0.9971), with an equation of the straight line of Abs=0.0057C (Figure 3) and which was reproducible. Similarly, the control drug amikacin was taken and dilutions were performed to obtain five standard solutions whose concentrations were within the range determined with glycine. In both cases, acceptable linearity, reproducibility and percentage of recovery were obtained.
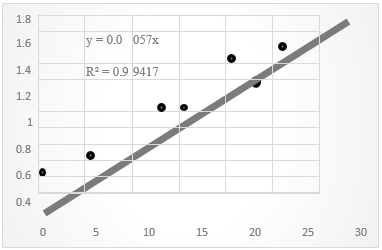
Figure 3 Calibration curve for glycine (reaction with Ninhydrin, measured at 540 nm; Y=absorbance, X=concentration in parts per million).
Once the calibration curves were obtained and the spectrophotometric method was validated, we proceeded with the quantification of expired drugs. The results obtained show that the concentration of the active ingredient tends to decrease as the expiration time increases; to establish this trend, the data on the % of active ingredient remaining vs. the expiration time were plotted (Figure 4).
A directly proportional trend of amikacin degradation was found as the expiration date of the drugs increases; however, the average correlation coefficient is explained because there are other factors besides the expiration date that affect the decomposition of the active ingredient; among them are the storage treatment received by the drugs and their exposure to environmental factors such as light and temperature that allow drugs with less expiration time to contain less active ingredient compared to drugs with longer expiration dates.
The authors declare that there are no conflicts of interest.
None.
None.

©2023 Mex-Álvarez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
2 7