eISSN: 2378-315X


Research Article Volume 11 Issue 2
1Kosair Charities Clinical and Translational Research Building, St. Louisville, USA
2Biostatistics and Bioinformatics Facility, University of Louisville, Louisville, USA
Correspondence: Shesh N Rai, Biostatistics and Bioinformatics Facility, University of Louisville, Louisville, KY, USA
Received: July 16, 2022 | Published: July 27, 2022
Citation: Lele R, Rai SN. Proposing a novel composite score as an outcome measure in a Phase II/III clinical trial with application to Type II Diabetes. Biom Biostat Int J. 2022;11(2):83-91. DOI: 10.15406/bbij.2022.11.00359
Background: In the complex disease, type II diabetes mellitus (T2DM), to study the effect of an intervention, using a single endpoint, such as glycated hemoglobin (HbA1c), and without stratified block randomization may not be Diabetic drugs may be toxic. Hence toxicity monitoring during a clinical trial is an important consideration.
Methods: In this article, we suggest conducting separate clinical trials for moderately (6.5% ≤ HbA1c < 5%) and severely (HbA1c ≥7.5%) diabetic patients to reduce heterogeneity due to disease burden at and baseline and expecting different responses withing each cohort, we propose a novel composite score for a Phase II/III clinical trial for testing therapeutic interventions in diabetic patients, and present sample size calculations required to conduct such a clinical trial. The composite score incorporates multiple outcomes together into a binary measure, based on HbA1c, which has not been used before in any of the clinical trials designed for diabetes. We also present the toxicity monitoring rules for the two diabetic populations.
Results: We expect to recruit higher number of patients for the moderately diabetic group than for the severely diabetic group. Using one or two interim analyses does not significantly change the required sample size
Conclusions: For the moderately diabetic patient group, an allocation ratio of 1:1 is advised and for the severely diabetic patient group, an allocation ratio of 1:2 is advised with a greater number of patients recruited on the experimental treatment Since clinical trials for T2DM are carried out over longer periods of time, we suggest using O’Brien-Fleming (OBF) alpha-spending function with two interim analyses.
Keywords: composite score, Phase II/III clinical trial, type II diabetes mellitus, Interim analysis, continuous toxicity monitoring, sample size
Type II diabetes mellitus (T2DM) is a lifestyle disorder typically associated with a combination of obesity with hypertension, elevated triglycerides, fasting hyperglycemia, low density lipoprotein (LDL) cholesterol (LDL) and reduced high-density lipoprotein (HDL) cholesterol.1 Additionally, T2DM is a prevalent metabolic disorder which is characterized by an imbalance in blood glucose level, high blood pressure along with a sedentary lifestyle that can cause major health risks.2 Glycated hemoglobin (HbA1c) that is a measure of chronic glycemia in diabetes patients has been proposed in literature to diagnose diabetes and identify people at risk.3 HbA1c is a commonly used measure to study glycemic control in clinical trials for diabetes.4 But T2DM is a complex disease and hence, using a single endpoint (such as HbA1c) in these studies may not reveal important characteristics of the disease and the effects of therapeutic interventions. Hence using composite endpoints has been recommended in literature.4 In the present study, we propose a novel clinical trial design that incorporates a composite score based on different measures using HbA1c. Prior clinical trials suggest using HbA1c > 6.5% (48 mmol/mol) as diagnostic criteria for patients with diabetes.3,5–7 To conduct clinical trials for diabetes, generally, all patients having HbA1c greater than the threshold value of 6.5% or 7% are studied together8-12 irrespective of the level of seriousness of their disease.
In the present design, we suggest splitting the diabetic population into two groups such as ‘moderately diabetic’ (6.5% ≤ HbA1c < 7.5%) and ‘severely diabetic’ (HbA1c ≥ 7.5%) based on their HbA1c levels and conducting separate clinical trials for the two groups. We present sample size calculations required for conducting separate clinical trials for the moderately diabetic and severely diabetic patients using a binary composite score that we have developed, and incorporating interim analysis (using Pocock and O’Brien-Fleming (OBF) alpha spending functions13 which ensures that the investigation of an experimental drug is sufficiently warranted. Also, we present continuous toxicity monitoring rules which can serve as guide for investigators to stop a trial in case a drug becomes overly toxic at any stage of patient recruitment.
Research design and methods
In this study, using HbA1c as the primary endpoint, we classify a patient population as ‘moderately diabetic’ if their HbA1c levels are between 6.5% and 7.5% and another population as ‘severely diabetic’ if their HbA1c levels are greater than 7.5%. For these two populations, we design two separate Phase II/III clinical trials with the aim of reducing their HbA1c levels to less than 6.5% for moderately diabetic group and less than 7% for severely diabetic group. Each clinical trial will be run for 6 months and there will be three follow-up visits after the baseline measurement visit as follows:
V1: baseline measurement
V2: first post-baseline measurement – month 2
V3: second post-baseline measurement – month 4
V4: third post-baseline measurement – month 6
1.We propose a novel composite score for a Phase II/III clinical trial design which is defined based on the following three outcome measures: (1) longitudinal outcome, (2) time-to-event outcome and (3) multinomial outcome. A detailed description for these outcomes is provided below.
2.Longitudinal outcome (X): The longitudinal outcome measures the average change in HbA1c values from multiple post-baseline timepoints and the baseline value in each individual. We define X as follows:
X = average difference between the baseline HbA1c value and post-baseline value
Thus, average change =
Time-to-event outcome (T): The aim of a diabetic drug is to reduce HbA1c levels. Hence, the event of interest is reduction in HbA1c levels, and we wish to model the time taken for achieving this reduction. We define T as follows:
T = time required for a severely diabetic patient to become moderately diabetic and for a moderately diabetic patient to become pre-diabetic
Since the study is run for 6 months, patients will be censored at 6 months.
Multinomial outcome (W): The multinomial outcome measures the actual HbA1c value post treatment. We define W as follows:
W = final HbA1c value post treatment
Here, we define the following three groups: pre-diabetic group (5.7 < HbA1c < 6.5%), moderately diabetic group (6.5% ≤ HbA1c < 7.5%) and severely diabetic group (HbA1c ≥ 7.5%).
While X is calculated using all the three post-baseline measurement values, T and W are calculated using only the last post-baseline measurement at 6 months.
X, T, and W will be calculated for each patient in the experimental and standard treatment arms. Now, we define X’, T’ and W’ for each individual in the two treatment arms as follows:
X’ = 1 if X ≥ 0
= 0 if X < 0
T’ = 1 if T ≤ 6 months
= 0 if T > 6 months
W’ = 1 if W < 6.5% for moderately diabetic patients or if a moderately diabetic patient becomes pre-diabetic
= 1 if W < 7% for severely diabetic patients or if a severely diabetic patient becomes moderately diabetic
= 0 otherwise
Based on X’, T’ and W’ defined above, we define binary composite score Y as follows:
Y = 1 if X’ = 1, T’ = 1 and W’ = 1 simultaneously
= 0 if X’ = 0 or T’ = 0 or W’ = 0
Y is calculated separately for each patient and the success rate is then calculated for the group based on Y.
Y = 1 denotes success in the clinical trial and Y = 0 denotes failure in the clinical trial.
Y = 1 means the experimental treatment ensures that average post-baseline HbA1c values are less than the baseline HbA1c values, the experimental treatment helps in reducing HbA1c values within 6 months post treatment, and the HbA1c values for patients in moderately diabetic group treated on the experimental treatment reduce to less than 6.5% and the HbA1c values for patients in severely diabetic group treated on the experimental treatment reduce to less than 7%.
Patients may, sometimes, experience a progressive disease in which their HbA1c levels go on increasing beyond the baseline value after receiving experimental treatment. An adverse event observed because of increase in HbA1c will be considered a failure.
Let = proportion of successes in group i; i =1, 2.
Thus, success rate ( ) will be defined by the proportion of patients which result in a composite score (Y) of 1.
For the standard treatment group, we assume a success rate of 25% in the moderately diabetic group and we assume a success rate of 40% in the severely diabetic group. For moderately diabetic patient group receiving experimental treatment, we expect an improvement in the success rate of at least 15% when compared with the standard treatment. Likewise, for severely diabetic patient group receiving experimental treatment, we expect an improvement in the success rate of at least 20% when compared with the standard treatment. We calculated these success rates based on the values presented in Jendle et al. for the AWARD-1, AWARD-2, AWARD-3, AWARD-4 and AWARD-5 studies.8
Stratification factors
Figure 1A provides a schematic of the different factors that may be related to development of T2DM. Some of these factors are grouped as mediating factors/hidden factors and others are grouped as moderating factors/covariates. Mediating factors are such factors that will change when experimental treatment is administered thus altering HbA1c values. Hence, mediating factors cannot be used for stratification in a clinical trial as they are volatile and may change as a patient’s condition improves or worsens. The mediating factors associated with diabetes are systolic blood pressure8,14-31 hypertension14,15,32 cholesterol14,15 triglycerides14,15 fasting glucose8,14,15 total fat33 etc.
Moderating factors are fixed factors, also known as covariates. Such fixed factors associated with diabetes are race,14 gender,14,15,32 smoking status,14,15 country,8 baseline HbA1c8,15 background medication use8,14 age14,15,32 waist circumference14 weight8,14 BMI15,32 etc. Since moderating factors remain fixed even as a patient’s condition improves or worsens, we can use these factors for stratification.
There is more than 25% prevalence of T2DM in the U.S. population aged ≥ 65 years.34 Males are more susceptible to certain forms of T2DM while females may be more susceptible to T2DM depending upon the stage of reproductive life.35 Diabetic patients tend to have a higher BMI.36 Thus, in the present study, we have used the following three stratification factors with two levels each: age, gender, and BMI.
Figure 1B presents a schematic representation of the study population and stratification factors. We have used 3 stratification factors, i.e., age, gender, and BMI with 2 levels each. Age is categorized as ‘< 65 years’ and ‘≥ 65 years’, gender has two levels ‘Male’ and ‘Female’, and BMI is categorized as ‘< 25 kg/m2’ or ≥ ‘25 kg/m2’. Thus, the study involves 6 strata in the moderately diabetic and severely diabetic groups.
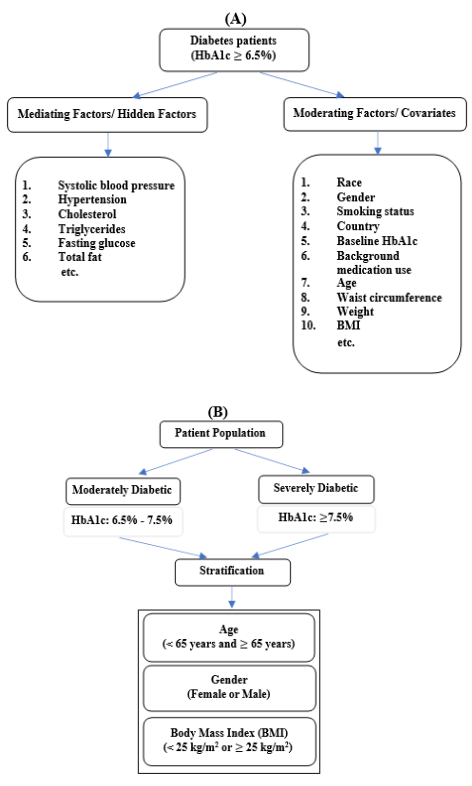
Figure 1 Stratification factors and study design incorporating stratification;
(A) Mediating and moderating factors for diabetes patients &
(B) Study Population with Stratification factors
Study design
Figure 2 presents the study design for conducting the current Phase II/III clinical trial. As shown in Figure 2, we present sample size considerations for the moderately diabetic patient group using randomization ratio of 1:1 and for severely diabetic patient group using randomization ratio of 1:2, power = 90%, = 0.025 and two interim analyses with futility (1/3, 2/3, Final). For comparison purposes, we also present sample sizes obtained using designs with one interim analysis. Interim analyses carried out in clinical trials help in knowing early on if a treatment is worth investigating. However, interim analyses lead to inflation in type I error rate and hence, a type I error adjustment is necessary13 for which we use Pocock and OBF methods. In the Pocock procedure, interim looks are equally spaced and the total type I error is equally distributed in each of the interim looks.37 In the O’Brien-Fleming procedure, unlike Pocock procedure, boundary values decrease over time.38 This ensures the trial does not stop early. OBF procedure allocates a conservative boundary in the early stages and boundary values at the final stage are close to the fixed-sample design.38
The baseline success rate for the moderately diabetic patient group is assumed to be 25% which is expected to be improved by 15% after being treated using the experimental therapy. Let P0 and P1 denote the proportion of success in the standard arm and in the experimental treatment arm based on their composite scores respectively. We present sample size calculations using P0 = 25% and P1 = (30%, 35%, 40%, 45%, 50%, 55%, 60%). The baseline success rate for the severely diabetic patient group is assumed to be 40% which is expected to be improved by 20% after being treated using the experimental therapy. We present sample size calculations using P0 = 40% and P1 = (45%, 50%, 55%, 60%, 65%, 70%).
Toxicity measures
Medications or drugs used to cure diseases can be toxic for the human body if taken in more than prescribed amounts. Diabetic drugs can be classified as hypoglycemic and antihyperglycemic drugs.39 These are the drugs prescribed to diabetic patients to help reduce blood sugar levels. Oral medicines for diabetes contain sulfonylureas which are associated with hypoglycemia which is a major symptom of toxicity due to diabetic drugs.39 Metformin which is a biguanide is a commonly used diabetic drug.39. However, if consumed beyond prescribed levels, it can cause considerable gastrointestinal adverse effects and potentially lactic acid acidosis in severe cases.39
As described before, common medications for diabetes include sulfonylureas and they carry a risk of hypoglycemia.40 However, if these drugs interact with other medications that a patient may be taking, it may lead to the inhibition of metabolism of sulfonylureas and increase systemic exposure which can cause unintentional sulfonylurea toxicity.40 Hepatotoxicity or toxic liver disease which is also known as toxic hepatitis is another form of toxic effect that has been observed in diabetic patients receiving drug therapies.41
Thus, toxicity monitoring in diabetes trials is an important consideration. If many toxicities are observed, the clinical trial must stop since it is unethical to expose patients to toxic medications. Therefore, we developed a toxicity monitoring rule which has been presented in Table 1.2 & 2.2. Continuous monitoring of toxicity ensures patient safety at every step of patient accrual.
Standard treatment |
Experimental treatment |
||
Component 1 |
X’ = 1 if X ≥ 0 |
||
= 0 if X < 0 |
|||
Component 2 |
T’ = 1 if T ≤ 6 months |
||
= 0 if T > 6 months |
|||
Component 3 |
W’ = 1 if W < 6.5% for |
||
moderately diabetic patients |
|||
= 1 if W < 7% for severely diabetic patients |
|||
= 0 otherwise |
|||
Composite Score |
Y = 1 if X’ = 1, T’ = 1 and |
|
|
= 0 if X’ = 0 or T’ = 0 or W’ = 0 |
(sum of all Y values |
(sum of all Y values in |
|
|
in the standard group) |
the experimental group) |
|
|
|
||
|
|
||
Proportion of |
|
|
Table 1 Definition of composite score and response variable
Interim Analyses
For the moderately diabetic group as well as the severely diabetic group, two interim analyses are recommended. We have presented results for both these groups using one and two interim analyses.42 We present results using Pocock and OBF alpha-spending functions. The measure for futility is also included in the study design.
One interim analysis
After the first interim analysis, half of the patient population was evaluated for efficacy using boundary for p-value as 0.011 and for the final look, this boundary was 0.025. These were the boundaries using Pocock method. The corresponding boundaries using OBF method are as follows: first look: 0.000 and final look: 0.025.
Two interim analyses
After the first interim analysis, using Pocock method, one-third of the patient population was evaluated for efficacy using boundary for p-value as 0.011, for the second look, it was 0.019 and for the final look, this boundary was 0.025. The corresponding boundaries using OBF method are as follows: first look: 0.000, second look: 0.006 and final look: 0.025.
Results for moderately diabetic patients
Table 1.1 presents sample sizes required to conduct a Phase II/III clinical trial on a group of moderately diabetic patients for testing a standard therapy against an experimental therapy. N1 represents the number of patients treated on the standard arm and N2 represents the number of patients treated on the experimental treatment arm. The power was assumed to be 90% at a significance level, = 0.025. Treatment allocation ratio was 1:1 and a one-sided test was used. Sample sizes were calculated using one and two interim analyses. We suggest using two interim analyses and OBF boundary which results in a total sample size of 424, i.e., an equal allocation of 212 patients in each of two treatment arms. Compared to one interim analysis, we require a marginally larger sample when conducting two interim analyses.
Pocock alpha-spending function |
OBF alpha-spending function |
|||||
P0 = 25% |
P0 = 25% |
|||||
P1 |
N1 |
N2 |
Total |
N1 |
N2 |
Total |
One Interim Analysis |
||||||
30% |
2015 |
2015 |
4030 |
1680 |
1680 |
3360 |
35% |
526 |
526 |
1052 |
439 |
439 |
878 |
40% |
241 |
241 |
482 |
201 |
201 |
402 |
45% |
138 |
138 |
276 |
115 |
115 |
230 |
50% |
89 |
89 |
178 |
74 |
74 |
148 |
55% |
62 |
62 |
124 |
52 |
52 |
104 |
60% |
45 |
45 |
90 |
37 |
37 |
74 |
Two Interim Analyses |
||||||
30% |
2267 |
2267 |
4534 |
1770 |
1770 |
3540 |
35% |
592 |
592 |
1184 |
462 |
462 |
924 |
40% |
271 |
271 |
542 |
212 |
212 |
424 |
45% |
155 |
155 |
310 |
122 |
122 |
244 |
50% |
100 |
100 |
200 |
78 |
78 |
156 |
55% |
69 |
69 |
138 |
54 |
54 |
108 |
60% |
50 |
50 |
100 |
39 |
39 |
78 |
Table 1.1 Required sample size for moderately diabetic patients with allocation ratio of 1:1 and one and two interim analyses using pocock and O’Brien Fleming (OBF) boundaries
In a Phase I trial (3+3 design) in which a drug is tested for toxicity, a true toxicity rate of 33% (1 toxicity out of 3 recruited patients) is commonly assumed. We have introduced a toxicity rate of 25% as a more conservative measure. (This can also be looked at as a true toxicity rate for a 4+4 design assuming 1 toxicity out of 4 recruited patients which amounts to a true toxicity rate of 25%.) Table 1.2 presents the toxicity boundaries obtained using probability of toxicity as 0.25, = 0.05, power = 90%, an allocation ratio of 1:1 using two interim analyses and a sample size of 271 based on Pocock alpha spending function.
Maximum number of subjects |
Number of subjects with toxicities |
Maximum number of subjects |
Number of subjects with toxicities |
Maximum number of subjects |
Number of subjects with toxicities |
1 |
1 |
79 |
32 |
179 |
63 |
2 |
2 |
82 |
33 |
183 |
64 |
3 |
3 |
85 |
34 |
186 |
65 |
5 |
4 |
89 |
35 |
189 |
66 |
6 |
5 |
92 |
36 |
193 |
67 |
8 |
6 |
95 |
37 |
196 |
68 |
10 |
7 |
98 |
38 |
199 |
69 |
13 |
8 |
101 |
39 |
203 |
70 |
15 |
9 |
104 |
40 |
206 |
71 |
17 |
10 |
107 |
41 |
209 |
72 |
20 |
11 |
111 |
42 |
213 |
73 |
22 |
12 |
114 |
43 |
216 |
74 |
25 |
13 |
117 |
44 |
219 |
75 |
27 |
14 |
120 |
45 |
223 |
76 |
30 |
15 |
123 |
46 |
226 |
77 |
33 |
16 |
127 |
47 |
230 |
78 |
35 |
17 |
130 |
48 |
233 |
79 |
38 |
18 |
133 |
49 |
236 |
80 |
41 |
19 |
136 |
50 |
240 |
81 |
44 |
20 |
140 |
51 |
243 |
82 |
47 |
21 |
143 |
52 |
247 |
83 |
49 |
22 |
146 |
53 |
250 |
84 |
52 |
23 |
149 |
54 |
254 |
85 |
55 |
24 |
153 |
55 |
257 |
86 |
58 |
25 |
156 |
56 |
260 |
87 |
61 |
26 |
159 |
57 |
264 |
88 |
64 |
27 |
163 |
58 |
267 |
89 |
67 |
28 |
166 |
59 |
271 |
90 |
70 |
29 |
169 |
60 |
|
|
73 |
30 |
173 |
61 |
||
76 |
31 |
176 |
62 |
||
Table 1.2 Continuous toxicity monitoring using probability of toxicity = 0.25 and α= 0.01.
From Table 1.2, we observe that in initial stages of the trial, if we observe 5 toxicities after 5 patients have been recruited and treated, the trial must stop since in such a case, the toxicity boundary of 25% has been exceeded.
Results for severely diabetic patients
For severely diabetic patients, we use an allocation ratio of 1:2 with a power of 90% and = 0.025. Here, as well, a one-sided test was used. We present results using both Pocock and OBF methods for sample size calculation.
Table 2.1 presents required sample sizes for allocation ratio of 1:2 using one and two interim analyses. From Table 2.1, we observe that to achieve the recommended increase in success rate from 40% to 60%, we require a total sample size of 303 if OBF procedure is used for calculating alpha- spending function and using 1:2 allocation ratio with two interim analysis. All the results for sample size calculations presented above were calculated using EAST software version 6.5.43 with different input parameters and assuming un-pooled estimate for the variance. The toxicity boundaries were calculated using R software version 4.0.2.
Pocock alpha-spending function |
OBF alpha-spending function |
|||||
P0 = 40% |
P0=40% |
|||||
P1 |
N1 |
N2 |
Total |
N1 |
N2 |
Total |
One interim analysis |
||||||
45% |
1844 |
3688 |
5532 |
1537 |
3074 |
4611 |
50% |
463 |
926 |
1389 |
222 |
444 |
1157 |
55% |
205 |
410 |
615 |
141 |
282 |
513 |
60% |
115 |
229 |
344 |
109 |
218 |
286 |
65% |
72 |
144 |
216 |
78 |
156 |
180 |
70% |
49 |
98 |
147 |
49 |
98 |
122 |
Two interim analyses |
45% |
2074 |
4148 |
6222 |
1620 |
3240 |
4860 |
50% |
521 |
1042 |
1563 |
407 |
814 |
1221 |
55% |
231 |
462 |
693 |
180 |
360 |
540 |
60% |
129 |
258 |
387 |
101 |
202 |
303 |
65% |
81 |
162 |
243 |
64 |
128 |
192 |
70% |
55 |
110 |
165 |
43 |
86 |
129 |
Table 2.1 Required sample size for severely diabetic patients with one and two interim analyses using Pocock and O’Brien Fleming (OBF) boundaries
Table 2.2 presents the toxicity boundaries obtained using probability of toxicity as 0.25, = 0.05, power = 90%, an allocation ratio of 1:2 using two interim analyses and OBF alpha spending function. As given in Table 2.1, the maximum number of subjects to be recruited using the previously mentioned design parameters is 129 in group 1 (standard treatment group) and 258 in group 2 (experimental treatment group). Hence, the maximum number of subjects used here is 258 and continuous toxicity monitoring rule was generated to establish toxicity boundaries.
Maximum number of subjects |
Number of subjects with toxicities |
Maximum number of subjects |
Number of subjects with toxicities |
Maximum number of subjects |
Number of subjects with toxicities |
1 |
1 |
76 |
31 |
173 |
61 |
2 |
2 |
79 |
32 |
176 |
62 |
3 |
3 |
82 |
33 |
179 |
63 |
5 |
4 |
85 |
34 |
183 |
64 |
6 |
5 |
89 |
35 |
186 |
65 |
8 |
6 |
92 |
36 |
189 |
66 |
10 |
7 |
95 |
37 |
193 |
67 |
13 |
8 |
98 |
38 |
196 |
68 |
15 |
9 |
101 |
39 |
199 |
69 |
17 |
10 |
104 |
40 |
203 |
70 |
20 |
11 |
107 |
41 |
206 |
71 |
22 |
12 |
111 |
42 |
209 |
72 |
25 |
13 |
114 |
43 |
213 |
73 |
27 |
14 |
117 |
44 |
216 |
74 |
30 |
15 |
120 |
45 |
219 |
75 |
33 |
16 |
123 |
46 |
223 |
76 |
35 |
17 |
127 |
47 |
226 |
77 |
38 |
18 |
130 |
48 |
230 |
78 |
41 |
19 |
133 |
49 |
233 |
79 |
44 |
20 |
136 |
50 |
236 |
80 |
47 |
21 |
140 |
51 |
240 |
81 |
49 |
22 |
143 |
52 |
243 |
82 |
52 |
23 |
146 |
53 |
247 |
83 |
55 |
24 |
149 |
54 |
250 |
84 |
58 |
25 |
153 |
55 |
254 |
85 |
61 |
26 |
156 |
56 |
257 |
86 |
64 |
27 |
159 |
57 |
258 |
87 |
67 |
28 |
163 |
58 |
|
|
70 |
29 |
166 |
59 |
||
73 |
30 |
169 |
60 |
||
Table 2.2 Continuous toxicity monitoring using probability of toxicity = 0.25 and α= 0.01.
From Table 2.2, we observe that in initial stages of the trial, if we observe 11 toxicities after 17 patients have been recruited and treated, the trial must stop since in such a case, the toxicity boundary of 25% has been exceeded.
The focus of this study was to introduce a novel composite score and present sample size calculations and toxicity monitoring rules for a Phase II/III clinical trial for T2DM. We expect an improvement in the power and efficiency of a trial using the composite score defined here when compared with trials that involve a univariate or a multivariate outcome. The novelty of this research is a) the stratification of diabetes population into moderately and severely diabetic groups and conducting separate trials for the two populations which has never been done before, and b) introducing a composite score that incorporates three different types of measurements which is beneficial because it considers different outcomes without having to inflate or adjust the type I error.
Type II diabetes is a highly prevalent disorder which is associated with lifestyle factors such as diet, low levels of physical activity, smoking etc. as well as physical factors such as obesity, high blood pressure and cholesterol etc. Owing to the fact that so many factors may affect an individual’s chance of developing diabetes, some studies suggest using composite endpoints instead of a single endpoint in order to carry out clinical trials for therapies for treating diabetes.44
Diabetes is a widely studied disease. Many different outcome measures as well as associated factors have been studied in literature. The study by Aitken et al suggests connection between saliva biomarkers and diagnosis of diabetes.32 The study by Bork-Jensen et al suggests that glucose tolerance is associated with differential expression of microRNAs in skeletal muscle.33 The article by Zafar et al describes the association of circulating angiogenic stem cells in T2DM with glycemic control and endothelial dysfunction.45
In the present study, we use HbA1c as the outcome measure. However, another measure called the estimated average glucose (eAG) has been suggested in literature and is recommended to be reported in addition to HbA1c by the Ameican Diabetes Association.46 eAG can be calculated using HbA1c.47,48 Thus, future studies can be designed using eAG or both HbA1c and eAG.
In this article, we have used HbA1c as an endpoint of interest and have developed a composite score which can be used to design a clinical trial for diabetes. We suggest splitting the study population into ‘moderately diabetic’ and ‘severely diabetic’ as these two populations exhibit different characteristics and hence, need to be treated differently. In a typical Phase II or Phase III trial, the emphasis is on establishing safety and efficacy of an experimental drug. In the present study, we suggest incorporating continuous toxicity monitoring in the study design because diabetic drugs have been proven to have some toxicity as they are consumed consistently over a long period of time during the lifetime of a diabetic patient. There are many mediating and moderating factors associated with diabetes. However, mediating factors cannot be used for stratification for the purpose of a clinical trial. In the present study, we suggest using age, gender, and BMI for stratification of the study population each with two levels. Thus, we have 6 strata and two groups of populations treated using an experimental therapy and compared with standard therapy.
Interim analysis is an important consideration that we have included in the present study. Clinical trials for diabetes are carried out over longer periods of time such as 6 months or a year. During this time, if we continuously monitor the efficacy of the drug, it can be beneficial to the patients because we can decide whether to continue a trial as it is showing required efficacy or stop for futility. Sample size calculations play a crucial role while designing a clinical trial. This study presents sample size calculations for a clinical trial for two groups of diabetic patients using two interim analyses and a composite score as the primary endpoint.
We have calculated required sample sizes to conduct clinical trials for testing the effectiveness of the experimental drug against the standard drug using various input parameters. This manuscript closely follows methods suggested in literature for similar Phase II/III trial design settings.49
If large trials are planned, using a multicenter trial may be a good approach as diabetes is a widely prevalent disease and patient accrual at different sites would not be difficult to carry out. While carrying out a multicenter trial, ‘center’ must be used as a stratification factor in addition to the factors mentioned in the present study. The advantage of using a multicenter trial would be that we will be able to get information from a larger number of subjects in a limited amount of time.
For studies conducted for targeting moderately diabetic group of patients, we suggest designing a clinical study design using 1:1 allocation ratio for treatments with both groups having 212 subjects to ensure that the statistical tests detect a significant difference of at least 15% between the success rates of the standard treatment compared with the experimental treatment and achieves 90% power. We suggest using O’Brien-Fleming alpha-spending function in this case with two interim analyses. The Pocock method results in a slightly larger sample size and this method could be used if we are conducting a larger trial where we require faster results. Thus, in case of global trials, Pocock boundaries can be used. OBF boundaries have been recommended in the present study as diabetes trials are carried out over longer periods of time. Also, the improvement in HbA1c as well as the success rates obtained using the composite score is a slow process and thus, OBF is the better option. Pocock recommends stopping a trial early and may not give reliable results with adequate sample sizes. Thus, we recommend using OBF for the present trial.
The allocation ratio of 1:1 was chosen for moderately diabetic group because this group is not a very high-risk group and hence the same number of patients can be treated on the experimental therapy and the standard therapy. However, the allocation ratio of 1:2 was chosen for the severely diabetic group because this is a high-risk group, and we want to treat higher number of patients on the experimental therapy as the experimental therapy is expected to have a 20% higher success rate than the standard therapy.
Continuous toxicity monitoring was incorporated in the design as diabetic drugs have been proven to be toxic to the human body in overdose situations. Also, if multiple therapies are prescribed to a diabetic patient, the drug-drug interactions could be toxic as well.50 Many approved diabetic drugs have been withdrawn due to adverse effects caused by drug interactions.50 Some examples of drugs withdrawn due to drug interactions include Terfenadine, mibefradil, and cisapride.50 Thus, toxicity monitoring is an important aspect to be considered when designing a Phase II/III clinical trial for diabetes. In the present trial, based on Table 1.2, we observe that if 271 subjects are recruited in the moderately diabetic group, the maximum acceptable number of subjects experiencing toxic events is 90. Beyond this, the investigational drug would not be acceptable due to toxicity concerns. Similarly, based on Table 2.2, we observe that if 258 subjects are recruited in the severely diabetic group, the maximum acceptable number of subjects experiencing toxic events is 87, beyond which the experimental drug should be declared toxic.
If a single center trial is planned with a smaller group of patients, conducting a Phase II trial is the better approach. Due to limited resources in this case, it is advisable to conduct a single center Phase II trial with reduced power such as 80% and testing a one-sided hypothesis since the expectation in such a trial is that the experimental therapy is effective. As against this, if a large multicenter trial is planned, designing, and conducting a Phase III trial is the better approach.
For studies targeting the severely diabetic group of patients, we recommend designing a clinical design using 1:2 allocation ratio for treatments, with the group receiving the experimental treatment having double the sample size as the group receiving the standard treatment. We suggest allocating 101 subjects to standard treatment arm and 202 subjects to the experimental treatment arm, giving a total sample size of 303. We suggest using OBF boundaries with two interim analyses.
Using a composite score as suggested in this article can help design an efficient clinical trial that incorporates three types of outcomes, namely, time to reducing HbA1c, average decrease in HbA1c and whether the HbA1c levels for the moderately diabetic group decrease below 6.5% and those for the severely diabetic group decrease below 7%. This study also incorporates toxicity monitoring to ensure stopping the trial if the experimental drug proves to be excessively toxic.
Rachana Lele was supported by National Institutes of Health (NIH) grant 5P20GM113226, PI:
McClain. S. N. Rai was partly supported with Wendell Cherry Chair in Clinical Trial Research Fund,
multiple National Institutes of Health (NIH) grants (5P20GM113226, PI: McClain;
1P42ES023716, PI: Srivastava; 5P30GM127607-02, PI: Jones; 1P20GM125504-01, PI: Lamont;
2U54HL120163, PI: Bhatnagar/Robertson; 1P20GM135004, PI: Yan; 1R35ES0238373-01, PI:
Cave; 1R01ES029846, PI: Bhatnagar; 1R01ES027778-01A1, PI: States; 1P30ES030283, PI:
Sates), and Kentucky Council on Postsecondary Education grant (PON2 415 1900002934, PI:
Chesney).
The authors declare no conflicts of interest.

©2022 Lele, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
2 7