eISSN: 2378-315X


Research Article Volume 10 Issue 2
2Immunology Research Center, Tabriz University of Medical Sciences, Iran
1 Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Iran
3 Department of Immunology, Faculty of medicine, Tabriz University of Medical Sciences, Iran
4 Department of Cancer and Inflammation, Southern Denmark University, Denmark
5 Department of Microbiology& Immunology, School of Medicine, Ardabil University of Medical Sciences, Iran
6 Stem Cell and Regenerative Medicine Institute, Tabriz University of Medical Sciences, Iran 7 Department of Cellular and Molecular Biology, Faculty of Biological Science, Azarbaijan Shahid Madani University, Iran
Correspondence: Seyed Isaac Hashemy, Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Received: April 24, 2021 | Published: June 17, 2021
Citation: Vahidian F, Najafi S, Shahgoli VK, et al. Determining the clinical and experimental findings of people with COVID-19. Biom Biostat Int J. 2021;10(2):68-73. DOI: 10.15406/bbij.2021.10.00332
Introduction: Coronavirus disease 2019 (COVID-19) is responsible for severe acute respiratory syndrome. The present study planned to evaluate the accuracy of laboratory parameters and clinical findings in predication of the infected cases through RT-PCR.
Methods: All related laboratory tests of 180 cases (62.8% male and 37.2% female) with an average of 62 years old (45.3-69) were performed by several laboratory techniques. The obtained results as well as other clinical and biography findings of the patients during the treatment and recovery were compared and analyzed to find out the possible relationship among the considered parameters.
Results: The considered hematological and biochemistry factors were completely different in all cases regardless to their health and lifestyle condition, including being at ICU, being addict or not, whether having previous diseases or not and others.
Conclusion: COVID-19 has brought a huge burden to the health system and infected patients, especially those who have a background of chronic diseases or addicted to some drugs.
Keywords: COVID-19, laboratory experiments, clinical finding, ICU
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, Alkaline Phosphatase; Baso, Basophil; BS, Blood Sugar; CRP, C-reactive protein; COVID-19, severe acute respiratory syndrome coronavirus 2; CPK, Creatine Phosphokinase; Hb, Hemoglobin; Hct, hematocrit; ICU, intensive care unit; Lymph, Lymphocyte; LDH, lactate dehydrogenase; Mono, Monocyte; Neut, neutrophil; PLT, platelet; RBC Red, blood cell; WBC, white blood cell
COVID-19 as a member of Coronaviridae family has a single-stranded, relatively enveloped, and positive-sense RNA, and leads to some acute diseases in animal species, particularly humans.1 Coronaviruses are responsible for several diseases such as neurological, hepatic diseases, enteric, common cold and most importantly respiratory disorders in human species, though CoV-2 (SARS-CoV-2) or COVID-19 as the third zoonotic coronavirus in 21st century is the most dangerous virus that causes severe acute respiratory syndrome with high spread and fatality rates in the globe.2-4 The first report of an infected case with COVID-19 was recorded in 2019 (December) in Wuhan, China. This disease then extensively and rapidly spread throughout worldwide through human-to-human transmission and quickly converted to be a disaster and pandemic disease in the world due to the lack of effective therapeutic strategies. It has been reported that approximately 50 million of people were infected by this virus in more than 200 countries and near to 1 million lost their life. However, it is predicated that the more mortality will be probably high in low-income countries due to the inadequate laboratories and health facilities. Thus, this epidemic is introduced as a serious public health concern by the World Health Organization (WHO).5
There are mainly four structural proteins in this virus; (i) nucleocapsid protein, (ii) envelope protein, (iii) glycoprotein and (iv) matrix protein. It is demonstrated that the spike protein has an important role in invading of the virus to the host cells though the interaction with angiotensin-converting enzyme 2 (ACE2) as a common receptor in different human organs such as respiratory system, spleen, brain, kidney, lymph nodes, thymus and liver.6 The symptoms of COVID-19, similar to the other viral infection are non-specific and variable at initial stage, and commonly appear at the first or second week after the infection. In most infected patients, mild type of fever, fatigue, dyspnea and dry cough are common symptoms, though the sever and advanced respiratory disorders are marked for this virus in sensitive cases, therefore, it requires rapid and selective detection, intensive treatment and management. Although the mortality rate of high-risk group such as immunocompromised, elderly, hypertensive and diabetic is more than healthy people, young and healthy people are present in the fatality statistic. Therefore, early diagnosis of the infected cases not only can help to control its spread, but also the pre-treatment can be begun.7
Though molecular diagnosis is known as a gold-standard technique for COVID-19 detection, clinical characterization, and different laboratory parameters-hematological and biochemical factors- can be fundamental for the detection, monitoring of the treatment process and its related response to control this pandemic. Furthermore, obtaining these parameters in the infected cases can provide information to distinguish the high or low risk group for mortality and also enhance the awareness of clinical and epidemiological situational. Complete blood counts (CBC) is an example for hematological parameters that can be monitored as an inflammatory marker for the detection. This study aimed to study clinical, biochemical and hematological data in the infected patients with COVID-19.8,9
Study design
This study was conducted on 180 confirmed patients with COVID-19 who were hospitalized at Emam Reza Hospital (Mashhad, Iran) from February 1 to June 20, 2020. The infection of all patients was verified via quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR), chest computed tomography (CT) and respiratory specimens’ tests according to the introduced guideline by the National Health Commission. The laboratory parameters and clinical characteristics were analyzed in the patients according to the status of the hospitalized at the intensive care unit (ICU) or not, and also dead or recovered. This was officially approved by the Ethics Committee of Emam Reza Hospital, Mashhad University of Medical Sciences, Iran. All laboratory data and clinical characterizations were achieved from medical records. The laboratory tests and analyses were carried out within 5 days of admission.
Hematology indicates
All blood samples were collected from the COVID-19-infected patients by routine methods, and the related hematological factors were analyzed by applying of XS-1000i hematology analyzer (Sysemx, Kobe, Japan). The considered parameters in this section were hematocrit Hct, Hemoglobin (Hgb), Platelet, White blood cell count (WBC), Neutrophil, Lymphocyte, Red blood cell (RBC) and Mixed (monocyte+eosinophil+basophil).
Biochemical indices
Biochemical parameters were obtained by a Biochemistry Analyzer, Hitachi System (Tokyo, Japan). The studied parameters in biochemical experiments were Blood Urea Nitrogen(BUN), Creatinine, Blood Sugar(BS), Alkaline Phosphatase (Alk.p), Serum Glutamic Oxaloacetic Transaminase (SGOT), Alanine Aminotransferase (SGPT), Direct Bilirubin (Bil_Direct), Alanine Aminotransferase (SGPT), Total Bilirubin( Bil_Total ), Creatine Phosphokinase( CPK), Direct Bilirubin (Bil_Direct), Calcium Total (Ca_Total), Lactate Dehydrogenase (LDH), C-reactive protein (CRP), Potassium, Magnesium( Mg), Phosphorus and Sodium.
Statistical analysis
The obtained data were expressed as mean (SD) whether distributed in median or normal manner (IQR), and also compared with the Mann-Whitney U test or Student t-test, respectively. The categorical variables were also analyzed as count (%) by Fisher’s exact test (χ² test) among the considered groups. In order to explain the strength and direction of analyses that defined for finding the linear relationship among laboratory parameters, spearman correlation coefficients were applied. Furthermore, P-value <0.05 was statistically described and considered as significant. GraphPad Prism version 8.0.0 (Windows) and IBM SPSS Statistics software version 26.0 (USA) were implemented for statistical analyses.
Clinical characteristics of the studied patients, according to status of the hospitalized at ICU and not ICU, and dead or recovered patients
In this study, a total of 180 COVID-19-infected and hospitalized individuals with an age range of 45.3 and 69 (average~62 years old) was studied. Among all considered cases, more than 60% of the patients (113 individuals) were male and the remainder was female. In this investigation, the patients were classified into two main groups; dead cases (37 individuals) and recovered cases (143 individuals). Considering to the higher proportion of the dead cases at the older age group (59-79.5), it can be concluded that the highest mortality rate belonged to over 65 years old group.
The manifestation of some symptoms such as shortness and difficulties of breathing 14(70), fatigue 4 (20) and heart failure were considerably more common in the dead group in comparison with the recovered group.
Regarding to being at ICU or not, 22 of the cases, 68.2% male and 31.8% female, with an average age of 61 (42-75.3) were treated at ICU, while the others were admitted in other sections. Furthermore, it was shown that most of the number of ICU patients were older than non-ICU patients, and non-case was recorded at ICU with <15 years old. It was also demonstrated that the non-ICU patients had more symptoms of fever (58.3%), fatigue (16.7%), pneumonia 1 (8.3) in relative to the ICU patients (Table 1).
Status |
||||
Characteristics |
Dead |
Recovered |
ICU |
Not ICU |
(N=37) |
(N=143) |
(N=22) |
(N=158) |
|
Age |
||||
Median (IQR)- yr |
70 (59-79.5) |
61 (43-67) |
61 (42-75.3) |
62 (46-68.3) |
Age distribution, n (%) |
||||
<15 yr |
0 (0) |
1 (0.7) |
0 (0) |
1 (0.6) |
<15-49 yr |
4 (10.8) |
52 (36.4) |
7 (31.8) |
49 (31) |
50-64 yr |
12 (32.4) |
41 (28.7) |
5 (22.7) |
48 (30.4) |
>65 yr |
21 (56.8) |
49 (34.3) |
10 (45.5) |
60 (38) |
Sex, n (%) |
|
|
|
|
Male |
20 (54.1) |
93 (65) |
15 (68.2) |
98 (62) |
Female |
17 (45.9) |
50 (35) |
7 (31.8) |
60 (38) |
Fever, n (%) |
9 (45) |
30(50) |
7 (58.3) |
32 (47.1) |
Shortness of breath, n (%) |
14 (70) |
40 (66.7) |
8 (66.7) |
46 (67.6) |
Fatigue, n (%) |
4 (20) |
6 (10) |
2 (16.7) |
8 (11.8) |
Chills, n (%) |
1 (5) |
12 (20) |
1 (8.3) |
12 (17.6) |
Cough, n (%) |
7 (35) |
26 (43.3) |
3 (25) |
30 (44.1) |
Pneumonia, n (%) |
1 (5) |
3 (5) |
1 (8.3) |
3 (4.4) |
Smoke, n (%) |
4 (11) |
11 (18.3) |
1 (8.3) |
14 (20.6) |
Heart failure, n (%) |
12 (60) |
28 (46.7) |
6 (50) |
34 (50) |
Table 1 Clinical characteristics of the studied patients, according to status of dead and recovered, ICU and not ICU, and dead or recovered patients
Laboratory results of the studied patients, based on the status of dead or recovered patients and ICU or not ICU hospitalized patients
This research studied the hematological and biochemical parameters of the 180 infected cases with COVID-19. Hematological tests indicated that WBC numbers were 10.3 (7-14.7), 10.2 (7.15-13.1), 7.22 (4.88-9.83) and 7.30 (5.00-10.30) for the dead cases, ICU patients, recovered patients and non-ICU patients, respectively, which confirms that WBC numbers were higher in the dead cases and ICU patients compared to the others.
Furthermore, there was a reduction in lymphocytes in the dead and ICU cases, 8.1 (4.6-14.4) and 9.7 (4-15.4) respectively, in comparison with the recovered and non-ICU individuals, 16.8 (11.2-24.2) and 15.7 (10.5-22.9) respectively. Totally, it can be seen that the level of neutrophil was increased in all infected patients, though the dead and ICUA groups presented the higher levels compared to the others. The data of Mixed (mono+eso+baso) (%) also revealed a significant reduction in the dead patients, however, it was not changed in the recovered group. The considered cases revealed no remarkable changes in Hgb and RBC levels, but Hct level reduced in the both dead and ICU individuals. Interestingly, platelet level raised in the ICU cases, however, there was no alteration in its level in others. Moreover, the results indicated that the level of BS increased remarkably in the dead and ICU cases compared to the other groups, while the BUN factor raised in all studied cases, particularly in the dead and ICU patients.
Although, any change was detected in creatinine, SGPT, Alk-p and levels of the cases, the rate of SGOT remarkably increased in all patients. The study also showed that the amount of direct bilirubin elevated in the dead and ICU cases, but no alteration was identified in total bilirubin level in all groups. Furthermore, a significant rise was recorded in LDH and CPK levels in all cases, particularly dead group. Despite Mg which indicated an increase, the level of calcium, phosphorus, sodium and potassium elements were not changed in all cases. It should be notified that CRP rate elevated exponentially in all patients (Table 2) (Figure 1, Figure 2 and Figure 3).
Status |
|||||||
Laboratory Results |
Dead |
Recovered |
ICU |
Not ICU |
Reference Values |
||
(N=37) |
(N=143) |
p value |
(N=22) |
(N=158) |
p value |
||
White blood cell count, (x10^3/ul) |
10.3 (7-14.7) |
7.22 (4.88-9.83) |
<0.001 |
10.2 (7.15-13.1) |
7.30 (5.00-10.30) |
0.056 |
3.5 - 9.5 |
Lymphocyte (%) |
8.1 (4.6-14.4) |
16.8 (11.2-24.2) |
<0.001 |
9.7 (4-15.4) |
15.7 (10.5-22.9) |
<0.001 |
16 - 45 |
Neutrophil (%) |
86.5 (78.5-92.2) |
76.9 (67.9-83.6) |
0.003 |
86 (79.1-92.6) |
77.8 (68-84.1) |
0.067 |
45.5-73.1 |
Mixed (mono+eso+baso) (%) |
4.45 (3.35-5.60) |
6.35 (4.00-9.18) |
0.01 |
4.6 (3.05-6.88) |
5.80 (3.88-8.73) |
0.13 |
6-15 |
RBC (x10^6/ul) |
4.19 (3.47-4.75) |
4.47 (4.06-4.93) |
0.008 |
4.21 (3.31-5.01) |
4.43 (4.01-4.89) |
0.17 |
Male: 4.5 6 Female: 3.7 - 5.5 |
Hgb (g/L) |
11 (9.65-13.8) |
12.7 (11.5-14.3) |
0.00 |
12 (9.58-14.1) |
12.5 (11.2-14.1) |
0.51 |
Male: 13.5 - 17.5 Female: 12 - 15.5 |
Hct (%) |
33.7 (29.9-40.6) |
37.7 (34.1-41.4) |
0.008 |
35.9 (29.6-43.2) |
37.2 (33.6-40.9) |
0.382 |
Male: 45 52 Female: 37 - 48 |
Platelet, (x10^3/ul) |
172 (101-274) |
210 (166-289) |
0.03 |
142 (61.5-253) |
210 (164-287) |
0.02 |
150 - 450 |
Bs (mg/dL) |
178 (99-221) |
113 (91.5-171) |
0.709 |
183 (92-236) |
115 (92.5-171) |
0.566 |
75 - 115 |
Bun (mg/dL) |
68 (39-91) |
34 (24-46) |
<0.001 |
55.5 (39.3-75) |
34 (24.8-47) |
0.00 |
3.5 - 23 |
Creatinine (mg/dL) |
1.3 (0.9-1.7) |
1 (0.8-1.2) |
0.04 |
0.95 (0.72-1.65) |
1.00 (0.8-1.28) |
0.459 |
0.5 1.5 |
SGOT (U/L) |
41 (23.5-53.5) |
33 (27-43) |
0.76 |
34.5 (21.8-45.3) |
33.5 (25.8-50) |
0.75 |
5.0 - 31.0 |
SGPT (U/L) |
28.5 (23.3-38.5) |
30 (21-47) |
0.405 |
36 (21.8-62) |
27 (21.5-45) |
0.762 |
5.0 - 47.0 |
Alkp (U/L) |
195 (145-264) |
181 (142-233) |
O.198 |
190 (147-236) |
188 (146-243) |
0.73 |
258 |
Bil_Total (mg/dL) |
0.4 (0.2-0.4) |
0.4 (0.4-0.7) |
0.684 |
0.7 (04-1.35) |
0.5 (0.4-0.72) |
0.955 |
0.3 1.0 |
Bil_Direct (mg/dL) |
0.35 (0.2-0.625) |
0.2 (0.2-0.3) |
0.77 |
0.4 (0.15-0.5) |
0.2 (0.02-0.3) |
0.77 |
0.1 - 0.3 |
LDH (U/L) |
738 (541-927) |
536 (433-743) |
0.774 |
560 (451-876) |
578 (436-765) |
0.797 |
140 - 280 |
CPK (U/L) |
477 (138-816) |
272 (206-586) |
0.72 |
291 (273-308) |
274 (138-840) |
0.59 |
24-195 |
Ca_Total (mg/dL) |
7.65 (6.95-8.15) |
8.10 (7.3-8.50) |
0.318 |
7.4 (6.9-8) |
8.10 (7.40-8.60) |
0.118 |
8.8 10.5 |
Phosporus (mg/dL) |
3.65 (3.23-5.05) |
3.2 (2.8-3.75) |
O.749 |
3.5 (2.83-5.13) |
3.40 (3.00-3.75) |
0.30 |
3 - 4.5 |
Mg (mg/dL) |
2.29 (1.87-2.30) |
2.3 (1.85-2.49) |
0.909 |
2.29 (2.02-2.30) |
2.30 (1.77-2.55) |
0.754 |
1.3 - 2.1 |
Sodium (mEq/L) |
136 (132-139) |
136 (133-138) |
0.41 |
136 (133-139) |
136 (133-138) |
0.31 |
135-145 |
Potasium (mEq/L) |
4.35 (3.85-4.60) |
3.9 (3.70-4.40) |
0.038 |
4.4 (4.1-4.9) |
3.90 (3.70-4.40) |
0.001 |
3.5-5.3 |
CRP |
176 (134-214) |
65.1 (20.7-129) |
0.02 |
76.8 (12-207) |
82.8 (25.5-169) |
0.83 |
0-6 |
Table 2 Laboratory results of the patients, based on the status of the Dead or Recovered patients, and ICU or not ICU hospitalized patients
Heat map matrix of the correlations of the hematological and biochemical data in the dead and ICU patients
Heat map matrix was used to investigate the correlations of biochemical and hematological indices. As revealed in Figure 1, Figure 2 and Figure 3, BUN and Creatinine showed a considerable positive correlation (r=0.678, p<0.0001) in the dead cases. Also, there was a remarkable negative correlation between Lymphocytes (%) and Neutrophils (%) in the dead group. These results suggest the possible correlations among the considered factors as important laboratory data with prognostic approach.
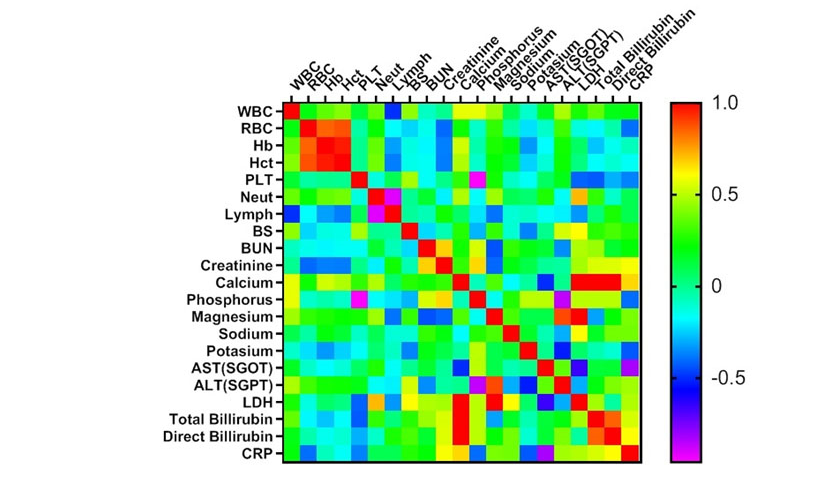
Figure 1 Heat map matrix of the correlations in dead patients (N=37). WBC: white blood cell, RBC Red: blood cell, Hb: Hemoglobin, HCT: hematocrit, PLT: platelet, Neut: neutrophil, Lymph: Lymphocyte, BS: Blood Sugar, BUN: blood urea nitrogen, AST: aspartate aminotransferase ALT: alanine aminotransferase, ALP: Alkaline Phosphatase, LDH: lactate dehydrogenase, CRP: C-reactive protein.
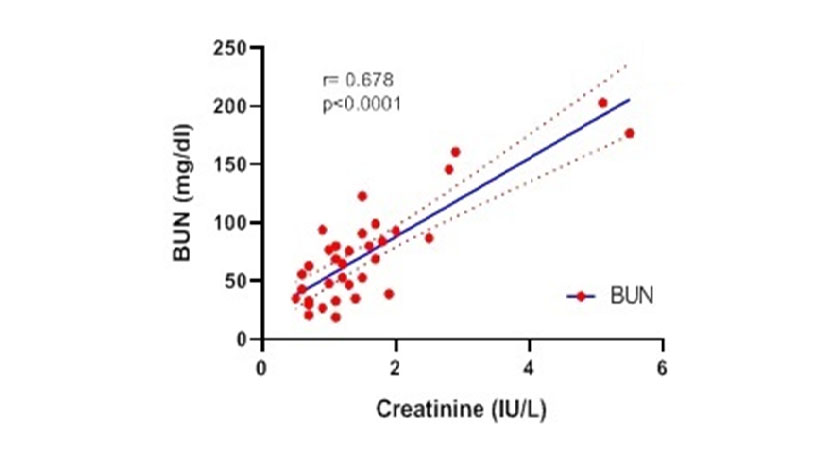
Figure 2 Spearman Correlation between BUN and Creatinine in dead patients. WBC: white blood cell, RBC Red: blood cell, Hb: Hemoglobin, Hct: hematocrit, PLT: platelet, Neut: neutrophil, Lymph: Lymphocyte, BS: Blood Sugar, BUN: blood urea nitrogen, AST: aspartate aminotransferase ALT: alanine aminotransferase, ALP: Alkaline Phosphatase, LDH: lactate dehydrogenase, CRP: C-reactive protein.
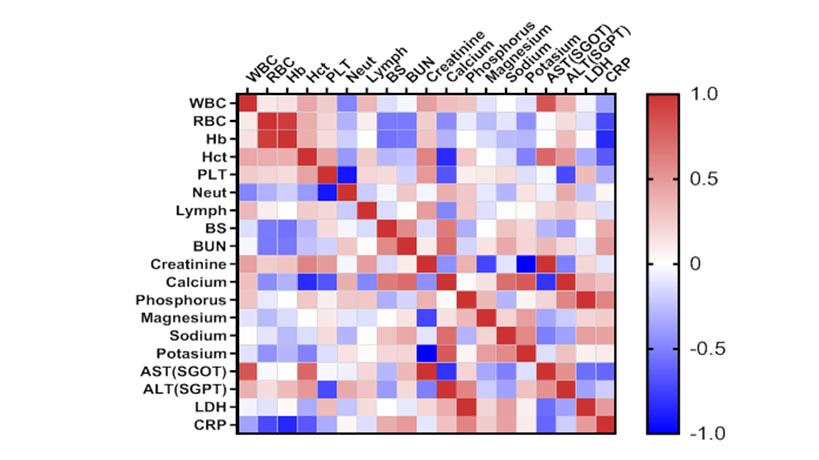
Figure 3 Significant negatively linear regression (r=-0.913, p<0.0001) between Neutrophils (%) and Lymphocytes (%) in dead patients. WBC: white blood cell, RBC Red: blood cell, Hb: Hemoglobin, Hct: hematocrit, PLT: platelet, Neut: neutrophil, Lymph: Lymphocyte, BS: Blood Sugar, BUN: blood urea nitrogen, AST: aspartate aminotransferase ALT: alanine aminotransferase, ALP: Alkaline Phosphatase, LDH: lactate dehydrogenase, CRP: C-reactive protein.
COVID-19, SARS-CoV2, has been emerged as a new zoonotic agent in December 2019, and is responsible for Severe Acute Respiratory Syndrome.10 The spread of COVID-19 infections has been rapidly increasing across the world since the first case recognized because of several reasons, including; (i) the high binding tendency of the spike glycoprotein for ACE2 receptors in human host cells, (ii)person-to-person transmission via respiratory droplets and aerosols under some circumstances, (iii) latent period, appearance asymptomatic infection with different atypical clinical signs and (iv) insufficient attention or treatment, particularly at the early stages.11-14 Though most infected patients with COVID-19 have mild symptoms as well as a good prognosis, a few of cases represent sever and developed acute respiratory distress syndrome (ARDS), pneumonia, the failure of multiple organs and pulmonary edema, which can potentially lead to death, therefore, they need to be hospitalized at ICU and receive oxygen therapy, especially those who have ARDS.15
The practical approach for this viral infection is to utilize the measures of personal precautionary to decrease the possible transmission risk and also early detection. The laboratory and clinical experimental data from the COVID-19-infected patients is able to provide a new aspect for better and more detection of this disease. This study confirmed that the two factors, age and gender, are basically crucial parameters in the death or recovery rates. In a study conducted by L Zhu and et al. reported that these two factors are essential for those who have diabetic disease and also infected with COVID-19. It was demonstrated that Diabetic-COVID-19 cases with older ages have higher mortality rate than young cases. In this research, it is also approved that the cases with previous respiratory or heart disorders are at higher risk of fatality compared to the others.16
The study also conducted the routine blood tests to compare different biochemistry and hematology factors of 180 confirmed-COVID-19 cases regardless their addiction condition to several drugs or alcohol, and hospitalized at ICD or not. It was found that the levels of WBC, LYM, NEU were considerably different between sever and mild group, which is the against of another report by Yong Gao et al, thought Huang et al. approved the reduction of WBC and lymphocytes counts in all cases.15,16
The data also indicated that the levels of LDH and CPK factors remarkably increased in all considered groups, particularly the dead and admitted at ICU groups. However, non-alteration was recorded in the rate of calcium, phosphorus, potassium and sodium. Further and more importantly, it was revealed that those cases addicted to various drugs or alcohol are more sensitive to COVID-19 infection.
The present ongoing pandemic, COVID 19, has brought a huge pressure to the health system in the world, and may be spread more extensively though human-to-human transmission. According to this study, the clinical and paraclinical signs of the infected patients with COVID-19 can be different, even in those who infected with the same virus, which can be positively associated with biochemistry and hematological factors. In other words, the severity of infection is defined as an independent and reliable predictor of the insufficient outcome. The recent findings revealed that the increased level of NLR factor and age can be potentially related to the severity of COVID-19 infection or its outcome.
This work was financially supported by a grant from the National Institute for Medical Research Development (NIMAD), Iran, and Mashhad University of Medical Sciences, Mashhad, Iran.
The authors declare that they have no conflict of interest.

©2021 Vahidian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
2 7