eISSN: 2378-315X


Research Article Volume 7 Issue 6
1Department of Chemistry, Beni?Suef University, Egypt
2Department of Botany & Microbiology, Beni-Suef University, Egypt
3Department of Hygiene, Beni?Suef University, Egypt
Correspondence: Mohamed EM Hassouna, Department of Chemistry, Faculty of Science, Beni?Suef University, Egypt,
Received: September 17, 2018 | Published: November 16, 2018
Citation: Hassouna MEM, Marzouk MA, Elbably MA, et al. Biosorption of iron by amended Aspergillus versicolor from polluted water sources. Biom Biostat Int J. 2018;7(6):502-513. DOI: 10.15406/bbij.2018.07.00253
Bacterial and fungal biomasses are economic natural adsorbents of metals because of negative surface charge and membrane composition. Hence, the present work aimed to investigate the use of Aspergillus versicolor (Ascomycota) as a biosorbent for the removal of iron from polluted water. Effects of pH, initial metal concentration, temperature and quantity of fungus on the biosorption capacity are also evaluated. The fungus is grown on Potato Dextrose Agar (PDA) plates amended with 100 ppm of Fe (II) ions and the standard spread plate method is applied. The plates are incubated at 27°C for 4 to 7 days, then the grown fungi are killed by adding 0.5 N NaOH, washed with distilled water, dried in an oven and finally grinded into fine powder. The optimum pH value for iron biosorption is 6. The rate of adsorption of iron by A. versicolor is very fast and reaching a maximum within 15 min. Maximum removal efficiency is observed at the biomass dosage concentration of 0.3 g A. versicolor.
Keywords: aspergillus versicolor, biosorption, economic, fungi, iron
Environmental pollution by heavy metals has become a question of public concern considering their hard consequences.1,2 Air, food, soil and water are narrated to be the media where heavy metals such as copper, cadmium, nickel, lead, zinc and iron are and iron are introduced into the environme.3 Presence of these metals in waste stream ground and drinking water is a very serious concern since these metal ions are toxic to various life form,4 that heavy metals and other constituents leach into the soil and damage the flora and fauna on the earth.5 Heavy metals are released from industrial operations such as electroplating, steel manufacturing, wood preservation, tanning, glass manufacturing and chemical processing which are eventually accumulating and circulating throughout the food chain causing severe problems to humans, animals and environment.
Iron is one of most spreading heavy metals making up to 5% of the earth"s crust, it is one of the major impurities that are commonly present in many water sources which cause several troubles for the human health. There are different methods for removal of iron from ground water, e.g., oxidation with chlorine and potassium permanganate treatment with limestone, liquid–liquid extraction, ion exchange, chemical precipitation, bioremediation, activated carbon, some filtering materials.6–9 These methods are generally of high cost with some disadvantages such as incomplete metal removal, much reagent and time consuming, energy requirements and generation of toxic sludge or other waste products that require further disposal or treatment.10 Biosorption using biomaterials has received considerable attention for detoxification of toxic heavy metals from wastewaters as they possess various merits such as low cost, high efficiency, minimization of chemical and or toxic sludge, regeneration of biosorbent, metal recovery etc.11–13
In metal polluted environments, microbial populations adapt themselves to high concentrations of heavy metals and become resistant to toxic concentrations.14 Algae, bacteria, fungi and yeasts have the ability to adsorb heavy metal ions.15,16 Bacterial and fungal biomasses are economic natural adsorbents of metals because of negative surface charge and membrane composition; the cell wall of the microorganisms essentially consists of various organic compounds such as carboxyl, acidic polysaccharides, lipids, amino acids and other components, extracellular polymeric substances present on the outer surface of some microorganisms that, also, contribute to biosorption of metal ions since those polymers contain negatively charged functional groups, such as carboxyl, phosphate and sulphate. Among microorganisms, fungi provide an effective, economic, and environmentally–friendly method of removing harmful wastes that accumulate as byproducts of industrial activities. The marine red alga Laurencia catarinensis was used in the biosynthesis of silver nanoparticles.17 Several studies have been forwarded towards the use of fungal biomass such as Rhizopus arrhizus, Saccharomyces cerevisiae and Aspergillus niger in the biosorption of heavy metals.18 It has been documented that Aspergillus spp are capable of the biosorption of metal ions, out of 23 fungi isolated from tannery effluent, 4 Aspergillus species namely A. terreus, A. tamarii, A. flavus and A. niger were selected for evaluating chromium tolerance and biosorption potential.In their review, (Siddiquee et al., 2015) reported that Aspergillus spp among other filamentous fungi were the most resistant to all the metals tested as cadmium, copper and nickel. Many species of genus Aspergillus have been used for heavy metal ion adsorption such as A. niger which has been used for the removal of Cr (VI),19,20 A. fumigatus to get rid of cadmium,21 A. versicolor for removal of lead ions from aqueous solutions Cabuk et al.,22 and cadmium,23 A. terreus for biosorption of lead, mercury and cadmium ions,24 A. cristatus for cadmium biosorption Hassan & Kassas25 and A. flavus has showed a higher affinity towards Fe(II).2
The main interest of the present study is to investigate the use of the highly ubiquitous Aspergillus versicolor resistant fungi powder as a bio sorbent for the removal of iron from aqueous solutions as a function of initial pH, initial metal concentration, temperature and quantity of the resistant fungus.
Chemicals
Ferrous ammonium sulphate (Aldrich), Hydrochloric acid (BDH), 1, 10 phenanthroline (Aldrich) (0.2%) solution of phenanthroline hydrochloride or hydrate (phen.) in 0.1 M HCl, tri sodium citrate (Aldrich) and hydroxylamine hydrochloride (Aldrich).
Reagents
Standard Iron (II) solution
(1000 ppm) Fe (II) stock solution is prepared by dissolving 0.7016 g of ammonium ferrous sulphate (NH4)2SO4.FeSO4.6H2O, (Aldrich, USA) in doubly distilled water (DDW) containing 5 mL conc. H2SO4 and accurately diluted to volume in 100 mL volumetric flask.
1, 10 Phenanthroline (0.2%)
In 100 mL vol. flask 0.2 gm of phenanthroline are dissolved in doubly distilled water (DDW) and diluted to the mark with 0.1 M HCl.
Tri sodium Citrate (10 %) solution: In 100 mL vol. flask, 10 g of tri sodium citrate are dissolved in DDW and diluted to the mark.
Hydroxylamine hydrochloride (10 %)
10 g of hydroxylamine–HCl are transferred to 100 mL vol. flask and dissolved in DDW, then completed to the mark.
Media preparation
Potato dextrose broth and agar media are prepared using 20 g of potato slices boiled with 100 mL of DDW for 30 min, the filtrate is obtained by strain through several layers of cheese cloth and then press the cloth to extract the remaining liquid. 2 g of dextrose are added, then make the filtrate up to 100 mL if necessary. For agar plates, 1.5 g of agar are added to this mixture and the flask was closed with cotton plug and aluminum foil and autoclaved for 20 minutes at 121ºC.
Preparation of Fungal Biosorbents
For fungal biosorption study, Potato Dextrose Agar (PDA) plates were amended with 100 ppm of Fe (II) ions and the standard spread plate method was performed. The fungus was incubated for 4 to 7 days at 27°C. )Wang & Chen 2009, after incubation period; the fully grown fungi (resistant metal fungi) are killed by adding 0.5 N NaOH in a conical flask containing the fungal mat and kept in a water bath for 15 minutes, the mat was washed with doubly distilled water for about 6–7 times till the pH reaches 7,the mat is then transferred to a sterile Petri dish and placed in hot air oven for 24 hours at 75°C.
The dried dead fungal mat was powdered to the smallest particle size using mortar and pestle, whereas it is known that the smaller the particle size, the larger the surface area. Biomass has been crushed to prevent particle aggregation for enhancing the biosorption capacity, then stored in sterile container for further study.
Instruments
UV/VIZ. Spectrophotometer (Shimadzu UV/VIZ. Perkin Elemer Lambada 3B Spectrophotometer using 1cm Quartz cell” was used for the determination of residual iron ions in the effluent of the samples after each adsorption process. Flame Atomic Absorption Spectrophotometer AA 240FS, Agilent Technologies, was used for rapid and conformational determination of iron ions; pH meter: the microprocessor pH meter BT 500 BOECO, Germany, which was calibrated against two standard buffer solutions at pH 4 and 9 was used for carrying out pH measurements and Mechanical Shaker with up to 200 rpm with speed control was used. The morphologies of the prepared samples were investigated using Scanning Electron Microscopy: TEM (JEOL–JEM– 100CX II).
Procedure
Spectrophotometric determination of iron
Sorption studies were performed in batch mode. After the biosorption process, the remaining iron ions in the solution are determined spectrophotometrically as follows . Transfer to 25 mL volumetric flask 0.5 mL of the 10 % hydroxylamine solution, 2 mL 10 % tri sodium citrate solution and 5 mL of the standard Fe (II) solution. The pH is adjusted to the range 3–4. Add 2.5 mL of 0.2 % 1, 10 phenanthroline solution, dilute to the mark with DDW and mix thoroughly. After 5 min the absorbance of the solution is measured at 512 nm against a blank.
Optimization of factors affecting the adsorption of iron
pH Optimization
To investigate the effect of pH on the adsorption % of iron from aqueous media by A. versicolor dried powder, aliquots of 25 mL containing 100 ppm of the metal ion are transferred to a set of 100 mL conical flasks each containing 0.3 g of the fungal powder, adjust the pH of each flask to a value in the range 1–10, respectively, using 0.1M NaOH and 0.1M HCl solutions and stir for 15 minutes. Centrifuge the contents of each flask and the supernatant solutions containing the residual iron contents are filtered and the concentrations of metal ions are measured by UV/VIZ spectrophotometry at 512 nm. Data are expressed as removal percentage of the initial concentration or specific biosorption q (mg/g), which is calculated by equation (1):
Where Ci is the initial iron concentration (ppm), Cf is the final concentration (ppm), Wt is the dose of sorbent (g) and V is the volume of solution (mL). The equation was applied in the pH range 1–10.The optimum pH is found to be 6 (Figure 1).
Biomass dose optimization
Aliquots equal to 100 ppm iron are transferred to a set of 100 mL conical flasks. Adjust the pH of each flask to the optimum value 6. Varying amounts of A.versicolor powder in the range 0.05–0.5g are added to each flask, respectively. The mixtures are stirred for 15 minutes, the residual iron content in the supernatant soln, separated by centrifugation is similarly determined spectrophotometrically.
Stirring time
A group of 100 mL conical flasks, each of which is loaded with the optimum biomass dose 0.3 g of A. versicolor powder and aliquots of 25 mL solution containing 100 ppm of iron at the optimum pH 6 and stirring is changed for different intervals of time (5–30 min.) for each flask in its role, respectively.
Metal ion concentration range
Applying the optimum conditions of the weight of A. versicolor powder, pH and stirring time in a group of flasks, aliquots of 25 mL solution containing varying concentrations of iron in the range 90–150 ppm are added to the flasks, respectively. The same procedure is applied and the residual iron content is determined from which the removal percent is calculated.
Desorption studies
Reusability of the adsorbent is tested by regenerating the spent adsorbent following the modified procedure of Chen et al.,26 The desorption process should yield the adsorbed metal in a concentrated form, restore the biosorbent close to the original state for effective reuse with undiminished metal uptake and physical changes or damages to it.27 The adsorbed iron ions on the adsorbent surface are treated with 25 mL 0.1M HCl and stirred for 1h. The amount of iron ions remained in the solution after filtration or centrifugation is measured using the recommended spectrophotometric method and the percentage desorption was calculated according to equation (2).
Where Cr is the released metal concentration and Ci is initially sorbed metal concentration.
Dead fungal biomass has been used in a number of studies because it is not affected by adverse operating conditions and can solve the environmental problems of high toxicity. Dead biomass achieves, relatively, best removal of heavy metals compared to the active biomass of the strain under the same conditions due to the pretreatment of the fungal biomass with heat enhancing its the stability, settling property and heavy metal uptake capacities of the biomass as previously reported Ratnasri & Hemalath.28 Under optimized conditions slight decrease in heavy metal ions noted in sorption potential from industrial effluents and wastewaters could be due to various impurities present in them in the form of anions i.e., SO42−, NO3−, and Cl− that may compete for binding sites on the fungal cell walls and then reduce the uptake of metallic ions from industrial wastewater using fungi.18 The dried powdered biomasses have been introduced as a new field of biotreatment technology, as effective and relatively simple methods for heavy metals recovery.29 Several studies have explained that inactive dried microbial biomass can passively bind metal ions via various physicochemical mechanisms.30 It has been suggested that the pretreatment by iron ions stimulates the surface characteristics groups by removing or masking them or by increasing the metal–binding sites.31 The use of fungal biomass is reported in literature for the removal of different heavy metals from wastewater e.g., out of 23 fungi isolated from tannery effluent, 4 Aspergillus species namely A. terreus, A. tamarii, A. flavus and A. niger were selected for evaluating chromium tolerance and biosorption potential Sugasini et al.,32 A. flavus was used by Verma et al.,2 for the removal of Fe(II), the uptake of chromium by Aspergillus foetidus,14 heavy metal biosorption using Aspergillus niger,15,16,19 heavy metal removal by fungus Mucor rouxii Khambhaty et al.,20 and biosorption of hexavalent chromium by dead fungal biomass of marine A. niger.33 The use of non–living biomass of A. versicolor in biosorption is more useful than bioaccumulation for water treatment, as the removal takes less time and continuous supply of nutrients is not required. A. versicolor was used previously for the removal of lead ions from aqueous solutions Cabuk et al.,22 and for Cr (VI), Cu (II), Ni (II) and RB reactive textile dye.29 A. versicolor is a one of the most common fungi, it is frequently found in soil, plant debris, marine environments, and indoor air environments.34 It is also among the most common of indoor molds.35,36 It is considered to be osmophilic as it is able to survive in solutions up to 30% NaCl or 40% sucrose.37
Different factors that affect the adsorption process have been extensively studied to improve the biosorption capacity Q (mg/g) of A. versicolor towards iron ions from aqueous solutions.
Optimum pH
Earlier studies showed that the pH value has been observed as one of the important parameters which control the heavy metal biosorption.38–40 The variation of adsorption rates at different pH values is dependent on both the metal chemistry in solution and the surface chemistry of the sorbent.41 Adsorption of iron by A.versicolor powder has been found to increase with the increase in pH value until attains its maximum at pH 6. The biosorption of the dried biomass is increased with the solution pH, due to the excess amounts of OH–ions within the solution, the binding sites on the fungal cell wall are negatively charged. The cell wall, as explained previously, is made up of several components such as carboxyl, carbonyl, alcoholic and amino groups which determines the biosorption ability based on its protonation or unprotonation nature. This exerted an influential attraction between active sites and positively charged ions. Thus, it is most possible that at low pH values, positively charged surface will not favor the binding with iron ions due to columbic repulsion. With increase in pH values, surface becomes more and more negatively charged and thereby favoring iron ion binding (Figure 1), (Table 1).
pH |
Std. deviation |
q (mg/g) |
1.00 |
0.1 |
4.1000±.05774 |
2.00 |
0.15275 |
8.1667±.08819 |
3.00 |
0.15275 |
12.1667±.08819 |
4.00 |
0.26458 |
16.2000±.15275 |
5.00 |
0.20817 |
20.1667±.12019 |
6.00 |
0.15275 |
22.1667±.08819 |
7.00 |
0.20817 |
18.1667±.12019 |
8.00 |
0.1 |
13.0000±.05774 |
9.00 |
0.11547 |
8.1333±.06667 |
10.00 |
0.1 |
3.1000±.05774 |
Total |
6.39491 |
12.5367±1.16754 |
Table 1 Effect of Initial pH on biosorption capacity of iron by A. Versicolor resistant powder, number of determinations N=3
Stirring time
The kinetics of adsorption describing the shaking time in the removal of iron is one of the characteristics defining the efficiency of the biosorption rate. The results indicate that maximum biosorption capacity takes place after 15 minutes where the uptake was 22.2 mg /g for iron ions. After this period, the equilibrium is reached (Figure 2 & Table 2).
Time of stirring |
Std. deviation |
q (mg/g) |
3.00 |
0.1 |
2.1000±.05774 |
6.00 |
0.15275 |
6.0333±.08819 |
9.00 |
0.15275 |
11.1667±.08819 |
12.00 |
0.15275 |
15.1667±.08819 |
15.00 |
0.15275 |
22.1333±.08819 |
18.00 |
0.2 |
20.2000±.11547 |
21.00 |
0.28868 |
12.1667±.16667 |
24.00 |
5.91805 |
13.8333±3.41679 |
27.00 |
0.1 |
4.0000±.05774 |
30.00 |
0.1 |
1.1000±.05774 |
Total |
7.25873 |
10.7900±1.32526 |
Table 2 Effect of stirring time on biosorption capacity of iron by A. Versicolor resistant powder, number of determinations N=3
Biomass dose
The increase in the biosorbent concentration from 0.05–0.5g results in extensive increase in the metal adsorption. The increase of the adsorption surface area and the availability of free adsorption sites help in the removal of iron. Maximum removal efficiency is observed at the biomass dosage concentration of 0.3 g A. versicolor, which achieves iron removal of 22.25 mg/g, after this concentration equilibrium is reached (Figure 3 &Table 3).
Dose |
Std. deviation |
q (mg/g) |
0.05 |
0.1 |
8.1000±.05774 |
0.10 |
0.1 |
12.1000±.05774 |
0.15 |
0.15275 |
14.1667±.08819 |
0.20 |
0.1 |
17.1000±.05774 |
0.25 |
0.1 |
20.0000±.05774 |
0.30 |
0.07638 |
22.3167±.04410 |
0.35 |
0.03215 |
22.2267±.01856 |
0.40 |
0.03215 |
22.2367±.01856 |
0.45 |
0.02517 |
22.2267±.01453 |
0.50 |
0.07638 |
22.2333±.04410 |
Total |
5.00218 |
18.2707±.91327 |
Table 3 Effect of biomass dose on biosorption capacity of iron by A. Versicolor resistant powder, number of determinations N=3
Effect of initial metal ion concentration
The interest in processes including heavy metal removing by microorganisms has increased considerably in recent years due to the biotechnological potential of micro–organisms in metals recovery,23 the maximum metal uptake was 22.2 mg/L at 90 ppm iron concentration, then iron removal decreases with the increase of iron concentration. At higher concentrations, the available sites for sorption become fewer in comparison with the molecules of solute present. Hence, the removal of metal ions is strongly dependent upon the initial solute concentration, so heavy metal tolerant microorganisms such as A. versicolor instinctively use defense mechanisms on exposure to metal stress (Figure 4 & Table 4).42
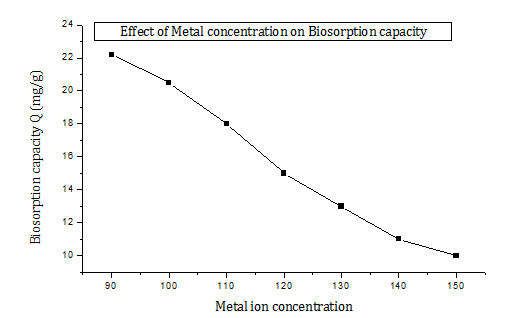
Figure 4 Effect of metal concentration on biosorption capacity of iron by A. versicolorresistant powder.
Metal concn. |
Std. deviation |
q (mg/g) |
15.00 |
0.06715 |
10.2000±.03877 |
90.00 |
0.1 |
22.1000±.05774 |
100.00 |
0.1 |
20.4000±.05774 |
110.00 |
0.15275 |
18.0333±.08819 |
120.00 |
0.15275 |
15.0333±.08819 |
130.00 |
0.1 |
13.1000±.05774 |
140.00 |
0.2 |
11.2000±.11547 |
150.00 |
0.14142 |
9.9000±.10000 |
Total |
4.39107 |
15.6952±.95821 |
Table 4 Effect of metal concentration on biosorption capacity of iron by A. Versicolor resistant powder, number of determinations N=3
Effect of temperature
Temperature is another most important factor in the biosorption process of metals. The increase in temperature has improved the iron biosorption rate and decreased the contact time required for heavy metal removal. The temperature of the adsorption medium is considered to be an important parameter for energy dependent mechanisms in metal removal using biosorbent. Maximum removal of iron is found to be 22.2 mg/g at a temperature equals to 31°C. Temperature affects the cell wall stability components, its configuration and ionization of chemical moieties and energy–independent mechanisms are likely to be affected due to temperature changes since the processes responsible for removal are largely physiochemical in nature.43 Similar results have been recorded in the bioaccumulation of Cu (II) and Cr (VI) by Streptococcus Equisimilis and Aspergillus sp (Figure 5 & Table 5).44
Tempr. |
Std. deviation |
q (mg/g) |
25.00 |
0.1 |
19.1000±.05774 |
27.00 |
0.15275 |
20.1667±.08819 |
29.00 |
0.20817 |
21.2333±.12019 |
31.00 |
0.1 |
22.4000±.05774 |
33.00 |
0.15275 |
21.1667±.08819 |
35.00 |
0.1 |
20.1000±.05774 |
37.00 |
0.15275 |
19.1667±.08819 |
39.00 |
0.1 |
18.4000±.05774 |
41.00 |
0.1 |
18.0000±.05774 |
Total |
1.39417 |
19.9704±.26831 |
Table 5 Effect of temperature on biosorption capacity of iron by A. Versicolor resistant powder, number of determinations N=3
The morphologies of the prepared fungi
Scanning electron microscope (SEM) provides information about the sample's surface topography and composition. SEM micrographs of dried biosorbent particles showed fairly regular spherical structures with an external surface which, although rather smooth, displayed a number of cracks likely to favor solute adsorption through enhanced diffusion to active sites (Figure 6).
Adsorption isotherm studies
It is more significant to study the adsorption behavior in order to provide predictions of the performance of the biosorption process under different operating conditions using the appropriate adsorption isotherm model. For solid–liquid adsorption system, the adsorption behavior can be described as adsorption isotherm model. The adsorption isotherm means the distribution of adsorbate molecules between the solid phase and the liquid one at equilibrium. Equilibrium is said to be reached when the concentration of adsorbate in bulk solution is in dynamic balance with that on the liquid adsorbate interface.
Langmuir adsorption isotherm
The equilibrium adsorption data for the concentrations of iron ions is fitted into the linear form of Langmuir’s isotherm equation, to determine the distribution of iron ions between the adsorbent and solution according to equation (3):
Where Ce is the equilibrium concentration of the iron ions in solution (mg L−1), Qe is the equilibrium concentration of iron ions on A. versicolor adsorbent (mg g−1), Qm and KL are Langmuir constants related to sorption capacity and the rate of adsorption respectively. Maximum adsorption capacity (Qm) is the monolayer capacity of the adsorbent (mg g−1) and KL is the Langmuir adsorption constant. A plot of Ce/Qe against Ce over the entire concentration range is a straight line with a slope of 1/Qm and intercept of 1/QmKL. The correlation coefficient (R2) values reported are very close to 1 indicating that the adsorption follows the Langmuir adsorption isotherm. The quality of Langmuir isotherm can be determined by the magnitude of a dimensionless constant RL known as the separation factor expressed in equation (4):
where Co is the initial concentration of the iron ions in mg L−1 and KL is the Langmuir constant described earlier. The adsorption process is favorable within the range 0 <RL<1, unfavorable when RL>1, becomes linear when RL= 1, and the process is irreversible when RL= 0. The value of RL is 0.047 for A. versicolor; hence the adsorption process is favorable (Figure 7), (Table 6).
Langmuir and Freundlich adsorption isotherm Parameters of iron ions by A.Versicolor |
||||
No. |
Langmuir isotherm parameters |
Freundlich isotherm parameters |
||
1 |
Qmax(mg/g) |
22.25 |
1/n |
0.8545 |
2 |
KL |
0.2 |
KF |
1.8 |
3 |
R2 |
0.961 |
R2 |
0.997 |
4 |
RL |
0.047 |
|
|
Table 6 Freundlich adsorption isotherm Parameters
Freundlich adsorption isotherm
The linear form of the Freundlich adsorption model equation (5):
Where Qe is the amount of iron ions adsorbed at equilibrium per gram of the adsorbent (mg g−1), Ce is the equilibrium concentration of iron ions in the solution (mg L−1), and Kf and n are the Freundlich adsorption model constants related to the adsorption capacity and adsorption intensity respectively. Log Qe was plotted against log Ce and a straight line is obtained giving the intercept of log Kf and the slope of 1/n. The reported numerical value of 1/n is less than1, (Figure 8 & Table 6).
Interferences
On the spiking of the iron authentic samples under the optimum conditions, with different concentrations of other metal ions e.g., Mn(II), Ni(II) and Cu (II), higher values for the removed iron were obtained, which means that there is no complete selectivity for the resistant A. versicolor powder towards iron ions. Perhaps, these results may be attributed to the use of the single recommended pH value for the adsorbate (pH 6) where some other metal ions, which may be copresent with iron in the matrix solution, are, also, coadsorbed at this pH value to the powdered biomass. Thus, the biosorption of Cu (II) by Aspergillus flavus was conducted at pH 8–9 and Pb (II) by Aspergillus niger at pH 4–5.4. Also, it was reported Ratnasri & Hemalatha28 that the pH value must be less than that for the precipitation of respective metal ions, thus the sorption of Fe3+ on some Aspergillus species was performed at pH 2.6 and for other species with Mn+2 at pH 8.
However, the amending of the fungus with iron ions resulted in obtaining the resistant A. versicolor powder which proved as efficient, low cost, available, economic biosorbent for removal of heavy metals in general, and especially, for the removal of relatively large amounts of iron.
Desorption studies
Effect of pH on desorption of iron
In strong acidic media at pH range (1.8–2.4) A.versicolor resistant powder showed high desorption percentages. With increasing the pH values desorption percentage decreases (Figure 9 & Table 7).
pH |
Std. deviation |
Desorption% |
1.80 |
0.15275 |
80.1667±.08819 |
2.40 |
0.1 |
79.9000±.05774 |
3.00 |
0.15275 |
73.1333±.08819 |
3.60 |
0.2 |
69.2000±.11547 |
4.20 |
0.20817 |
64.0667±.12019 |
4.80 |
0.15275 |
60.1333±.08819 |
5.40 |
0.20817 |
55.1667±.12019 |
6.00 |
0.32146 |
54.1333±.18559 |
6.60 |
0.15275 |
50.1333±.08819 |
7.20 |
0.15275 |
47.1667±.08819 |
7.80 |
0.20817 |
43.2333±.12019 |
8.40 |
0.1 |
39.9000±.05774 |
Total |
13.35395 |
59.6944±2.22566 |
Table 7 Effect of pH on desorption%, number of determinations N=3
Stirring time
The maximum desorption percentage is found to be 80 during the first 20 minutes, then gradually decreased. (Figure 10 & Table 8).
Stirring time |
Std. deviation |
Desorption% |
20.00 |
0.15275 |
80.1667±.08819 |
25.00 |
0.1 |
60.0000±.05774 |
30.00 |
0.20817 |
50.2333±.12019 |
35.00 |
0.15275 |
41.1667±.08819 |
40.00 |
0.28868 |
35.1667±.16667 |
45.00 |
0.15275 |
34.1333±.08819 |
50.00 |
0.1 |
33.1000±.05774 |
55.00 |
0.26458 |
32.2000±.15275 |
60.00 |
0.1 |
32.1000±.05774 |
Total |
15.88023 |
44.2519±3.05615 |
Table 8 Effect of stirring time desorption%, number of determinations N=3
Real samples
Water samples collected from Bahr Youssef canal, ground water, Ibrahemia canal and tap water from the districts in which drinking water plants mix the River Nile water with some ground water, are subjected to the adsorption procedure as illustrated previously and the residual iron is analyzed by two methods of finish viz., colorimetry and AAS (Table 9).
Collected samples |
Final concentration |
Std. deviation |
Recovery% |
Drinking water |
0.32 |
0.10263 |
86.1133±0.05925 |
Bahr Youssef Canal |
1.06 |
0.07638 |
80.8833±0.04410 |
Ground water |
1.36 |
0.1 |
82.2000±0.05774 |
Ibrahimia Canal |
1.72 |
0.1 |
81.6000±0.05774 |
Table 9 Concentration of Fe (II) on real water samples, number of determinations N=3
Colorimetry |
AAS |
||||||||
Parameter |
Optimum |
Mean |
Std. deviation |
Std. error |
q (mg/g) |
Mean |
Std. deviation |
Std. error |
q (mg/g) |
pH |
6.00 |
22.166 |
0.15275 |
0.0881 |
22.166±0.0881 |
22.100 |
0.10000 |
0.5773 |
22.100±0.5773 |
Time of stirring, min |
15.00 |
22.133 |
0.15275 |
0.0881 |
22.133±0.0881 |
22.2833 |
0.15275 |
0.0881 |
22.283±0.0881 |
Dose, g |
0.30 |
22.316 |
0.07638 |
0.0441 |
22.316±0.0441 |
22.050 |
0.18028 |
0.104 |
22.050±0.1040 |
Metal Concentration, mg |
90.00 |
22.1 |
0.1 |
0.0577 |
22.100±0.0577 |
21.933 |
0.05774 |
0.3333 |
21.933±0.3333 |
Temperature °C |
31.00 |
22.4 |
0.1 |
0.0577 |
22.400±0.0577 |
22.400 |
0.10000 |
0.0577 |
22.400±0.0577 |
Table 10 Summery of factors affecting the extraction of iron by A. Versicolor, number of determinations N=3
Biosorption of iron ion by A. versicolor resistant fungus is shown to be an effective bioremoval process. It could retain relatively high quantities of metal ions with increased capacity towards the adsorption of the amending metal ion, although its selective adsorption hasn’t completely achieved. The kinetics of biosorption is rapidly enhanced with temperature increase and acidic pH. Both Langmuir and Freundlich adsorption isotherm models are suitable for predicting the biosorption capacity mg/L of A.versicolor within the range of the studied variables.
None.
The authors declare no conflict of interest.
This article does not contain any studies with human participants or animals performed by any of the authors.

©2018 Hassouna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
2 7