
Research Article Volume 11 Issue 5
An analytical study of the critical values of response
rate in single-arm phase II clinical trial designs
Yi Liu,1 Sin-Ho Jung2 
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

1Department of Statistics, North Carolina State University, USA
2Department of Biostatistics and Bioinformatics, Duke University, USA
Correspondence: Sin-Ho Jung, Department of Biostatistics and Bioinformatics, Duke University, USA
Received: November 07, 2022 | Published: December 30, 2022
Citation: Yi L, Sin-Ho J. An analytical study of the critical values of response rate in single-arm phase II clinical trial designs. Biom Biostat Int J. 2022;11(5):178-183. DOI: 10.15406/bbij.2022.11.00374
Download PDF
Abstract
A single-arm phase II clinical trial is usually conducted for finding an appropriate dose-level and testing toxicity for an experimental cancer therapy in comparison to some historical controls, and is usually the most doable trial type due to the feasibility under limited budget, patient pool and medical conditions that can be met. We considered the standard setting of a single-arm two-stage phase II clinical trial, and investigated the patterns of critical values and sample sizes at both two stages of minimax and optimal designs under different design parameters, i.e., under different response rates of control and treatment, significant level of the test, and statistical power. We provided analytic derivations to the patterns we are interested under large sample approximation, and investigated them under finite ans small sample via a numerical study by considering extensively possible design parameters over a fine grid. We finally concluded that the critical values at different stages of the test are related to the sample sizes at different stages in a similar way for both optimal and minimax designs, but they also reveal some differences and reflected the nature of these two types of design.
Keywords: single-arm two-stage trial, optimal design, minimax design, critical value, response rate
Introduction
A phase II clinical trial is often conducted to find an appropriate dose-level and test toxicity for experimental cancer therapies in compare to some historical controls. This kind of trials usually requires small sample sizes due to ethical considerations, before proceeding to the subsequent phase of the trial for assessing the efficacy, outcomes and adverse effect in a larger group of subjects.1,2 At the same time, a single-arm design is often the most doable trial due to the feasibility under limited budget, patient pool and medical conditions that can be met. The number of written requests reported on 2021 of single-arm trials issued by the Food and Drug Administration (FDA) of the United States is an essential volume.3
Statistically, the most simple and traditional single-arm phase II clinical trial design-the single-stage design, is specified as follows. Given the values of design parameters
, that is, statistical significance level, power of the test, null response rate, and treatment response rate, respectively, we want to find a pair of positive integers
to specify the sample size and rejection boundary (or critical value, for rejecting
) of the phase II trial under some constraints, and usually we also have a upper bound of
specified subjectively, such as
to reflect the small-sample characteristic of a phase II trial.4 Then, starting from
for a fixed
(the minimal sample size needed), we can search over a grid the smallest integer
such that based on level
, we reject
if
and otherwise fail to reject
, that is,
where
is the CDF of
. Then if the power
where
is the CDF of
, the pair
is said to be the optimal design. If there is no
meet these conditions simultaneously, we continue to
. Thus, the optimal design minimizes the sample size among all designs satisfies the conditions. It is also easy to know that the optimal single-stage design under a parameter setting is unique.
In some scenario, two-stage designs are more likely to be considered. In a two-stage design, the phase II clinical trial is conducted via two stages sequentially, and whether to proceed to the second stage depends on the results from the first stage. A desired design under the specifications of some parameters is given by finding appropriate sample sizes and critical values of the response rate of both two stages. The two-stage design is more economical and ethical, which makes the trial stop earlier if there is no efficacy tested, and the data collected is more informative than that from a single-stage trial if we proceed to phase III.4 Similar to single-stage design, the two-stage design is a mathematically easy problem since it can be done over a finite grid once the parameters are specified. In this paper, the problem is stated and discussed under the design of a single-arm two-stage phase II clinical trial.
The remainder of the paper is organized as follows. Section 4 reviewed the statistical setup of the single-arm two-stage phase II trials and described the scientific question of interest. We reviewed the classical optimal and minimax designs and discussed an issue we are concerned about involved in these two designs, and briefly stated our hunch. Section 5 introduced a numerical analysis to confirm our hunch. Section 6 concluded the paper.
Single-Arm two-stage Phase II clinical trials
Setup
Consider the test
vs.
, where
is the response rate of a historical therapy,
is the response rate of the experimental therapy needs to be tested, and under our context,
for some
as a clinical meaningful difference. We conduct the test by two stages. Denote
with
as the sample size of the test at stage
. Denote
and
under
.
and
are independent.
We consider two types of stopping criteria when the phase II trial needs to be stopped after stage 1. First, we consider the futility (lower) stop only, which means we stop the trial after stage 1 only if we find the treatment is ineffective based on the result from stage 1, in other words, this happens when we find the positive response of stage 1
for a critical value
that requires us to reject the null hypothesis
under specified design parameters. Second, we consider both futility and superiority (upper) stops. The superiority stop means we also stop the trial when
for a critical value
that specifies the enough effectiveness of the treatment, i.e., because of the enough positive responses to the treatment, we can stop and claim the effectiveness of the treatment, and there is no need to conduct stage 2.
Specifically, the framework of finding specific designs of a single-arm two-stage phase II clinical trial is specified as follows. Under futility stop with design parameters
:
- Calculate
,
- Define probability of early termination (PET) under
:
and expected sample size (EN) under
:
- Among designs with
and
:
- Optimal design
minimizes EN
- Minimax design
minmizes
where
is the probability measure under
. The significant level and power can be calculate by
where
for
and
for
,
is the probability mass of
and
is the corresponding CDF,
. The algorithm of finding an optimal or minimax design is easy to implement via greedy searching over possible choices of
. Starting from a
, for
, the probabilities and EN aforementioned can be calculated, and so the corresponding designs can be found.
Under both futility and superiority stops with design parameters
:
- Calculate
- Define probability of early termination under
, and expected sample size (EN) under
,
, and then
- Among designs with
and
:
- Optimal design
minimizes EN
- Minimax design
minmizes
A user-friendly software for finding optimal and minimax designs of two-stage phase II clinical trials can be found at http://www2.cscc.unc.edu/impact7/CTDSystems.
What are we looking for?
Following the notations in Section 4.1, we are interested if there is any pattern(s) among the
values we found from the desired designs given the constrains on level, power, etc. In fact, Jung4 found a phenomenon that
,
and
by a few examples from the output of optimal and minimax trial designs, but has not investigated this in more details. We wanted to provide a formal analysis in the relationship of these numbers that an optimal or minimax design returns, given any specific setting of the design parameters, which we believe can provide some prior knowledge about what we can expect from a specific design, help us to understand more on the difference and similarity of minimax and optimal designs, and look at them from a new perspective.
First, we proved that under some regularity conditions and constraints by phase II clinical trials, and using large sample approximation,
,
, and
for some
, and
is a parameter that can be related at least to the response rates of control and treatment, i.e.,
and
. In other words, we may expect that the rejection criteria, i.e., the critical values
and
of the test are mainly determined by the response rates of control and treatment when the sample size is large. Details can be found in Appendix 7.1 for single-stage design and 7.2 for two-stage designs. Second, we used an extensive numerical study in Section 5 to confirm our hunch when the sample size is finite and small, and based on results from optimal and minimax designs.
Numerical analysis
Setup
We investigated the patterns of
over a fine grid of parameters
. We set
ranged from
with a small increment
, and
, where
. We consider
and
for all of these
and
values. We restricted the maximum of the total sample size
by 55 to reflect the small-sample-size characteristic of phase II trials. Finally, we considered both optimal and minimax designs under two stop criteria, i.e., (1) with futility stop only; (2) with both futility and superiority stops.
Under a parameter setting, we searched the (minimax or optimal) design
if the trial stops at futility stop of stage 1,
if the trial is allowed to stop at both futility and superiority stops of stage 1.
Based on the theoretical results in Appendix 7, we investigated: (1) under futility stop only, the trends of
(and
) for stage 1, and
(and
) for stage 2, i.e. we choose
only in our numerical experiment (based on some previous experience and we would like to confirm how if
is a good candidate for
); (2) under both futility and superiority stops, the trends of
(and
) and
(and
) for stage 1, and
(and
) for stage 2.
Results
For simplicity and because of the similarity of our findings in multiple cases, we only present the results under
, where
, as a representative, under both stop criteria and both designs. The complete results under all parameters we considered can be found in Appendix 8.
Figures 1& 2 show that for different
, the trends of ratio differences we are interested, i.e.,
,
and
. They indicate that overall, these differences are close to 0, and the difference is smaller on stage 2, i.e.,
, under both stopping criteria, which means
is a good approximation to
over the
we considered under this parameter setting. At the same time, we can observe that, the other two differences are also close to 0, but the differences under optimal design are overall closer to 0 than those of minimax design. The results for all other cases in Figure 3 to Figure 12 in Appendix 8.1 and 8.2 are similar to this case. We plotted all trends including differences in ratio and frequency. They also result in the same conclusion.
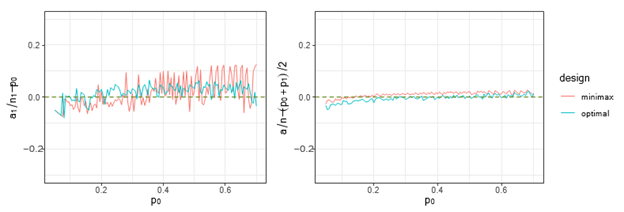
Figure 1 Trends of
and
under both optimal and minimax designs, when
and there is futility stop only.
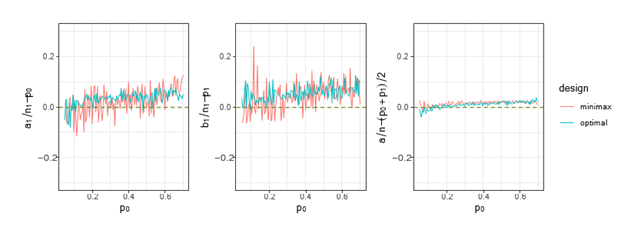
Figure 2 Trends of
,
and
under both optimal and minimax designs, when
and there are both futility and superiority stops.
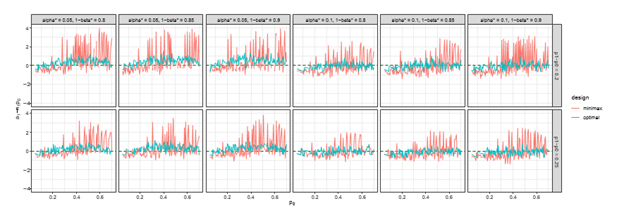
Figure 3 The trends of
over different choices of
, under both optimal and minimax designs, when there is futility stop only.
Table 1 calculates the summary statistics of the trends in Figures 1 &2 over different
(in total, there are 131
’s in each figure). We found from the mean that, these differences are very close to 0 on average, and thus the approximation we use is good. We found from the interquartile range (IQR) and standard deviation (SD) that they are larger for minimax design than optimal design for a same difference, thus the variation of the approximation on optimal design is smaller. Table 2 further provides all numerical results under this setting, i.e.
, including all design outputs, ratios and differences of interest. We present results over
but with increment
compared to
for the figures in order to maintain the concision and information provided by the table.
Concluding remarks
In this paper, we focused on single-arm two-stage phase II clinical trials, which is widely needed in reality. We specifically take the trends of critical values for rejecting null hypothesis at both two stages in a phase II clinical trial into consideration. Using both theoretical derivations under large-sample and numerical studies under finite and small sample, we confirmed that the critical values can be approximated and dominated by the total sample sizes and response rates of different stages. We also found that, this approximation is more obvious in optimal design than that in minimax design under the same design parameter setting. Our finding indicates that although minimax design provides less total sample size needed for a phase II trial, the property of an optimal design is closer to what we expect under large sample, and overall the optimal designs have more stable patterns on their relationships among critical values, sample sizes of both stages and response rates under null and alternative hypotheses.
Appendix: technical proofs
The numerical study in Section 5 confirms our hunch in Section 4.2 under finite (and actually small) sample. In this section, we provide some analytic proofs about the relationship between
and
when the total sample size
is large, using normal approximation, based on some regular assumptions (specified later).
Large sample approximation of single-stage phase II trials
In a single-arm single-stage trial, given a large
, we can find the approximate value of
using large sample approximation. We assume that there exist
and
such that
, which means the response rates satisfy the “positivity”, i.e., bounded away from 0 and 1. In reality, this means we assume that a treatment cannot be effective or ineffective to all subjects in the target population. Next, denote
, then under
:
(7.1.1)
as
, where
,
. Denote
by
. Given
,
and
satisfy
and
. So, under a large sample,
(7.1.2)
and
(7.1.3)
where
is the
-quantile of the standard normal distribution. From (7.1.2) and (7.1.3), we know
or
By plugging this in (7.1.2) or (7.1.3), we have
(7.1.4)
with
.
Note that both
and
are finite, and usually, in phase II clinical trials, we choose
so that
. Hence, if
, the weights in (7.1.4) are closer to
.
Large sample approximation of two-stage phase II trials
In the case of two-stage trials, we investigated in Section 5 by numerical studies under finite and small sample. Now, we provide an analytic proof assuming some regularity conditions about under large sample. Following notations in Section 4.1, note that under
:
(7.2.1)
as
, where
.
Under futility stop only
Under a large
and assume
, a constant, as
, the condition
is equivalent to
,
where
is the density of
, and
is the survival function of
under
. Note that
where
is the CDF of a standard normal distribution. Let
,
Note that there exists an
such that
since the lower limit of the above integral
.
Then,
(7.2.2)
Therefore, by (7.2.1) and (7.2.2), we know
(7.2.3)
where
is the
-quantile of the standard normal distribution. Thus, if we choose
(achievable usually since
should be small in practice), then
is finite, and thus
as
.
Next, we show the relationship among
and
. Let
and by independence,
under
. Thus, (7.1.1) holds for
. We know
and
.
One the one hand,
, and let
On the other hand,
and let
We note that there exist an
such that
for both
, thus the right hand side of the above two probabilities are conservatively equal to
under large sample. Now,
Thus,
(7.2.4)
with
. By choosing
, we have
and
, so both
and
are finite. Whether the weights in (7.2.4) are closer to 1/2 is a more complicated problem here than that in Section 7.1.
Under both futility and superiority stops
Following the same notations and assumptions made in Section 7.2.1, note that similar to the thoughts in Section 7.2.1, condition
is equivalent to
where
Thus, by choosing
, we can similarly show that
(7.2.5)
Thus,
.
Second, we note that
. Hence, the condition
is equivalent to
, thus
where
Then, by choosing
, we know that
(7.2.6)
Thus,
.
Finally, denote
, and we have that
,
and
. Let
and
where
is the CDF of the standard normal distribution, and thus choosing
and
, similar to the proof in Section 7.2.1, we have
(7.2.7)
where
.
Appendix: complete results of the numerical study
We provide figures of two types of trends. That is, trends in frequency (Figures 3 & 4) for investigating the trends of and , and trends in difference of ratio (Figures 5 & 6) for investigating the trends of
and
.
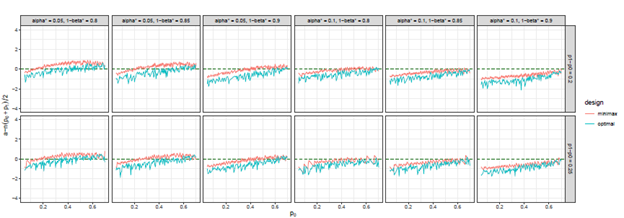
Figure 4 The trends of
over different choices of
, under both optimal and minimax designs, when there is futility stop only.
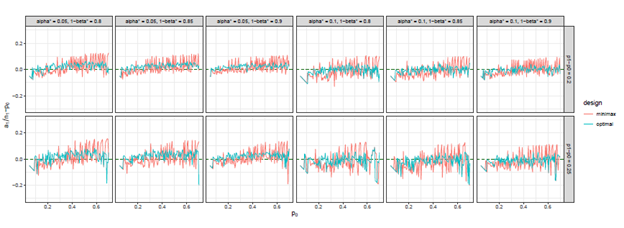
Figure 5 The trends of
(ratio) over different choices of
, under both optimal and minimax designs, when there is futility stop only.
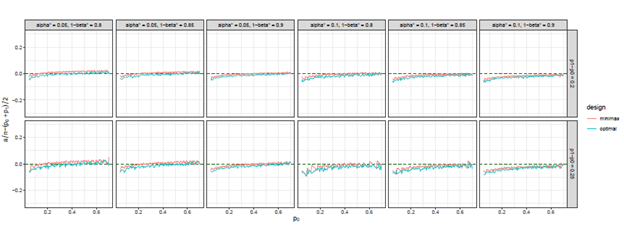
Figure 6 The trends of
(ratio) over different choices of
, under both optimal and minimax designs, when there is futility stop only.
Table 3– Table 8 present all numerical results, including the returns of designs, ratios, and differences of interest under all parameter settings in this numerical study. Specifically, we only present under
with an increment
compared to
in the figures, for maintaining the information and concision provided by the tables simultaneously. Each table represents a case of
.
Results under both futility and superiority stops
Same as Section 8.1, we provide figures of trends in frequency (Figure 7-Figure 9) for investigating the trends of
,
and
, and trends in difference of ratio (Figure 10-Figure 12) for investigating the trends of
,
and
.
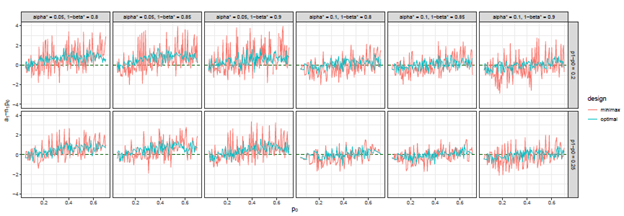
Figure 7 The trends of
over different choices of
, under both optimal and minimax designs, when there are both futility and superiority stops.
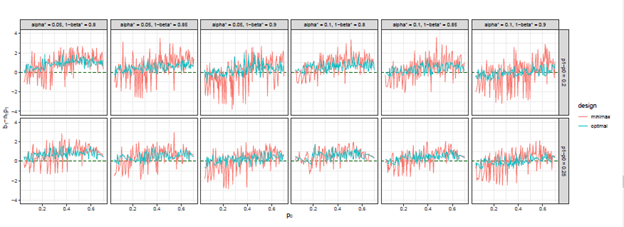
Figure 8 The trends of
over different choices of
, under both optimal and minimax designs, when there are both futility and superiority stops.
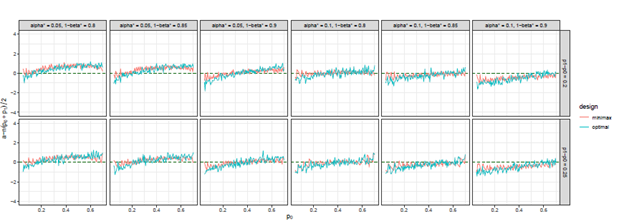
Figure 9 The trends of
over different choices of
, under both optimal and minimax designs, when there are both futility and superiority stops.
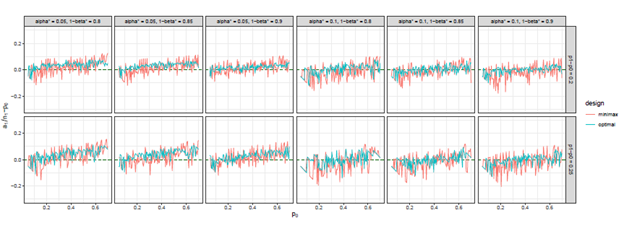
Figure 10 The trends of
(ratio) over different choices of
, under both optimal and minimax designs, when there are both futility and superiority stops.
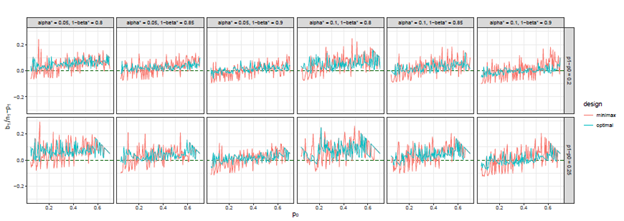
Figure 11 The trends of
over different choices of
, under both optimal and minimax designs, when there are both futility and superiority stops.
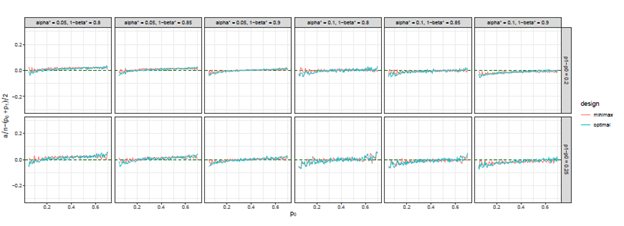
Figure 12 The trends of
(ratio) over different choices of
, under both optimal and minimax designs, when there are both futility and superiority stops.
Table 9-Table 14 present all numerical results, including the returns of designs, ratios, and differences of interest under all parameter settings in this numerical study. Specifically, we only present under
with an increment
compared to
in the figures, for maintaining the information and concision provided by the tables simultaneously. Each table represents a case of
.
Acknowledgments
Conflicts of interest
The authors declared that there are no conflicts of interest.
References

©2022 Yi, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.














