Advances in
eISSN: 2377-4290


Case Report Volume 15 Issue 1
Grandstaff VA Medical Center, Spokane WA 99205, USA
Correspondence: Len V Koh OD PhD, Mann-Grandstaff VA Medical CenterEye Clinic Building 30, 4815 N Assembly Street, SpokanevWA99205, USA, Tel 509-434-7032
Received: January 27, 2025 | Published: February 20, 2025
Citation: Koh LV, Coit-Makadia A, Gosnell CE, et al. Central retinal artery occlusion and PED after a prolonged coughing paroxysm. Adv Ophthalmol Vis Syst. 2025;15(1):7-11. DOI: 10.15406/aovs.2025.15.00476
Background: Central retinal artery occlusion is a rare but a severe ophthalmic emergency leading to permanent vision loss in most patients. It was identified over 160 years ago but effective treatment remains to be discovered.
Case report: A 77-year-old Caucasian American male presented to urgent care clinic with sudden loss of vision OS after a prolong coughing episode due to pulmonary fibrosis. After evaluation for stroke, he was sent to the eye clinic for further ocular examination. He was diagnosed with comorbid CRAO and pigment epithelial detachment (PED) OS. Subsequently, he was seen by a local retinal specialist who sent him to a local hospital emergency department for a more extensive stroke work up. After two-day of hospital stay, he was discharged in a stable condition and to continue care with primary care provider, retinal specialist, pulmonologist and cardiologist.
Conclusion: Although it is rare to encounter ocular emergencies in the eye clinic, but it can be in your chair; sometimes, two urgent conditions can show up simultaneously. Therefore, eye care providers should be prepared with a handy reference on what to do, and which team to activate to achieve optimal co-management to preserve vision and save life.
Keywords: central retinal artery occlusion, sudden loss of vision, pigment epithelial detachment, ocular emergencies, pulmonary fibrosis, stroke
Central retinal artery occlusion (CRAO) is a rare but a severe ophthalmic emergency leading to permanent vision loss in most patients. It was first identified as an embolic disease associated with endocarditis by Von Graefe in 1859.1 Subsequent ophthalmoscopic observations of CRAO were described by Schweigger in 1864.2 This condition typically affects individuals over 60 years old, with an incidence of approximately one per 100,000 people, and less than 2% presenting with bilateral involvement. CRAO is more prevalent in men and is associated with various risk factors, including thromboembolic disease, hypertension, smoking, hyperlipidemia, diabetes, and hypercoagulable states.3,4 The most common culprits are fibrin-platelet emboli and thrombi, followed by cholesterol-containing emboli or calcific detritus obstructing the central retinal artery lumen within a compromised ocular arterial circulation, leading to retinal infarction or ischemia.4,5 Notably, patients with CRAO have a significantly reduced life expectancy compared to age-matched individuals without the condition, 5.5-years versus 15.4 years.3 Furthermore, CRAO patients face an increased risk of death, stroke, and myocardial infarction both in the short and long term, suggesting the need for comprehensive multidisciplinary evaluation and ongoing systemic follow-up.6 Inflammatory vessel, arteritic, disease can artery occlusion but accounting for less than 5% of patients and can be effectively treated with steroids.7 The primary goal in managing CRAO is to minimize irreversible retinal tissue damage and restore visual acuity through prompt diagnosis and intervention. Despite extensive research on various aspects of CRAO, effective treatment for non-arteritic CRAO remains elusive.
This is a rare case report presenting a comorbid CRAO and pigment epithelial detachment (PED) after a bout of prolonged coughing paroxysm. Further, a short overview on the management of CRAO is shared.
A 77-year-old Caucasian American male presented with sudden loss vision in left eye during a lengthy episode of cough around 9:00PM. He had pulmonary fibrosis and coughed frequently He came to the urgent care clinic (UCC) at a VA medical center (VAMC) the next morning and was subsequently referred to the eye clinic. He did not have any previous symptoms of flashes or floaters, or any eye pain. There was not any neurological deficits such as impaired speech, facial weakness, arm or leg weakness. He denied of nausea or vomiting. He had no scalp tenderness or jaw claudication, and no new shoulder pain. His last eye exam at the VAMC was about two years ago with early cataracts and good vision in each eye. His problem list was lengthy: heart failure, back pain, chronic atrial fibrillation, coronary artery disease, interstitial lung disease, pulmonary fibrosis, and vertebral artery occlusion. His medication list includes albuterol, apixaban, rosuvastatin, furosemide, diclofenac topical, losartan, omeprazole and oxycodone.
Best-corrected visual acuity was 20/20- OD and light perception (LP) OS. Extraocular muscle motility was full without restrictions, confrontation fields were full OD, and none OS, and pupils were equal, round, reactive to light with severe afferent pupillary defect OS. Anterior segment findings showed corneal arcus 360, and mild cataracts OU, otherwise unremarkable, and intraocular pressures were 13 mmHg in both eyes via Goldmann applanation tonometry.
Dilated fundus examination revealed relatively normal fundus with cup-to-disc (C/D) ratio of 0.30 round OD (Figure 1A). The fundus photo of the left eye showed a pale posterior pole with swollen optic nerve head and macular cherry spot (Figure 1B). Ocular coherent tomography (OCT) macular cube scans showed a representative slice of normal retinal layers through the macula OD (Figure 2A), and a pigmented epithelial detachment and disrupted retinal layers extending temporally from the optic nerve head (ONH) OS (Figure 2B). Additionally, Figure 3 and Figure 4 showed the OCT optic disc cube 200x200 scan with swollen ONH OS, and OCT macular cube 512x128 scan with detached retinal layers OS, respectively.
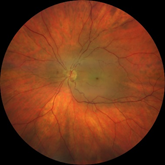
Figure 1B Fundus photo of patient’s retina showing pale posterior pole with swollen optic nerve head and macular.
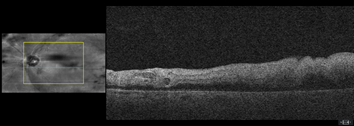
Figure 2B a slice of OCT macular cube showing pigmented epithelial detachment, and disrupted retinal layers OS.
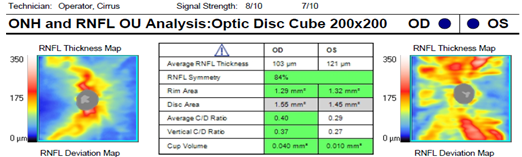
Figure 3 OCT ONH and RNFL OU analysis showed relatively normal optic nerve OD and swollen optic nerve OS.
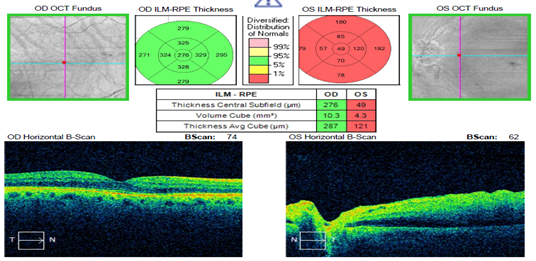
Figure 4 OCT Macular Cube 512x128 confirmed normal central thickness OD, and detached retinal layers OS.
No evidence of acute infarction, intracranial hemorrhage, cerebral edema, mass, mass effect, or midline shift was found by CT of the head. Head and neck CTA showed occluded right vertebral artery extending from the origin to the distal V2 segment, approximately 40% proximal left cervical ICA stenosis, interstitial and subpleural fibrosis with mildly enlarged mediastinal lymph nodes, like prior chest CT.
What to do with an ocular emergency in this case: CRAO and PED. The patient was walked back to the UCC with warm hand-off for stroke work up. The urgent care physician noted that the patient was on apixaban and did not feel that this was an apparent stroke. He was instructed to return to UCC or follow-up with an emergency department if any other stroke like symptoms arise. He was discharged in stable condition, and subsequently referred to a local retinal specialist for retinal management. The retinal specialist confirmed CRAO OS and sent patient to emergency room (ER) of a local hospital. His blood lab results indicated that he had low red blood cells, hemoglobin, high fibrinogen, high CO2, high BUN/Creatinine ratio, and high erythrocyte sedimentation rate (Table 1). The patient was seen at the ER two days later with the following diagnoses: left proximal ICA stenosis, chronic atrial fibrillation, interstitial lung disease, chronic GERD, and severe shoulder arthritis. After two days of hospital stay, he was discharged at stable condition to continued care with his primary care provider, pulmonologist and cardiologist, and follow up with the retinal specialist in 3 months.
|
Recent results (from the past 24 howls) CBC with differeintia result |
Value |
Ref Range |
|
White Blood Cells |
6.76 |
3.8 - 11.0 K/uL |
|
Red Blood Cells |
3.97 (L) |
4.20 - 5.70 M/uL |
|
Hemoglobin |
12.8 (L) |
13.2 - 17.0 g/dL |
|
Hematocrit |
39.3 |
39.0-50.0% |
|
MCV |
99.0 |
80.0-100.0fL |
|
MCH |
32.2 |
27.0 – 34.0 pg |
|
MCHC |
33.6 |
32.0 - 35.5 gfdL |
|
RDW-CV |
14.2 |
11.0 - 15.5% |
|
RDW-SD |
51.8 |
fL |
|
Platelet Count |
160 |
150 - 400 K/uL |
|
MPV |
9.8 |
9.3- 12.7 fL |
|
Absolute Neutrophils, Automated |
4.24 |
K/uL |
|
% Neutrophils |
62.7 |
40.0 - 75.0 % |
|
% Lymphocytes |
24.3 |
15.0 - 48.0% |
|
% Monocytes |
7.7 |
0.0 - 12.0 % |
|
% Eosinophils |
3.8 |
0.0 - 7.0 % |
|
% Basophils |
1.2 |
0.0 - 2.0 % |
|
% Immature Granulocytes |
0.3 |
0.0 - 1.0 % |
|
Absolute Neutrophils |
4.24 |
1.90 - 7.40 K/uL |
|
Absolute Lymphocytes |
1.64 |
1.00 - 3.90 K/uL |
|
Absolute Monocytes |
0.52 |
0.00 - 0.80 K/uL_ |
|
Absolute Eosinophils |
0.26 |
0.00 - 0.50 K/uL |
|
Absolute Basophils |
0.08 |
0.00 - 0.10 K/uL |
|
Absolute Immature Granulocytes |
0.02 |
0.00 - 0.03 K/uL |
|
% nRBC |
0.0 |
0.0 - 1.0 per 100 WBCs |
|
Absolute nRBC |
0.00 |
K/uL |
|
PPT Result |
Value |
Ref Range |
|
aPTT |
34 |
26 - 36 seconds |
|
Fibrinogen Result |
Value |
Ref Range |
|
Fibrinogen |
426 (H) |
211 – 419mg/dL |
|
Comprehensive Metabolic Panel Result |
Value |
Ref Range |
|
Na |
142 |
135 - 145 mmol/L |
|
K |
4.6 |
3.5 - 5.0 mmol/L |
|
Cl |
107 |
99 - 109 mmol/L |
|
CO2 |
30 (H) |
21 - 28 mmol/L |
|
Anion Gap |
5 |
5 - 16 mmol/L |
|
Glucose |
85 |
65 - 99 mg/dL |
|
BUN |
24 |
8 - 25 mg/dL |
|
Creatinine |
0.93 |
0.70-1.30 mg/dL |
|
Calcium |
9.6 |
8.5 - 10.2 mg/dL |
|
Albumin |
4.1 |
3.3-4.8 mg/dL |
|
Bilimbin Total |
1.1 |
0.2 - 1.1 mg/dL |
|
Total Protein |
7.8 |
6.1-7.8 g/dL |
|
AST |
34 |
10 - 45 U/L |
|
ALT |
21 |
10 - 65 U/L |
|
Alkaline Phosphatase |
80 |
35 - 115 U/L |
|
Globulin |
3.7 |
2.0 -4.0 g/dL |
|
Albumin/Globulin Ratio |
1.1 |
0.7 - 2.2 |
|
BUN/Greatinine Ratio |
25.8(H) |
10.0 – 24.0 |
|
eGFR |
85 |
>60 mL/min/1.73m2 |
|
Troponln I Result |
Value |
Ref Range |
|
Troponin I, High Sensitivity |
17 |
0 - 45 ng/L |
|
C-Reactive Protein Result |
Value |
Ref Range |
|
C-Reactive Protein |
0.8 |
0.0 - 1.5 mg-dL |
|
Sedimentation Rate Result |
Value |
Ref Range |
|
Elythrocyte Sedimentation Rate |
63 (H) |
0- 15 mm/hr |
|
Lipid Panel, with Dined LDL Result |
Value |
Ref Range |
|
Cholesterol |
126 |
<=199 mg/dIL |
|
Triglycerides |
44 |
0 - 149 mg/dL |
|
HDL |
56 |
>=40 mg/dL |
|
Chol/HDL Ratio |
2.3 |
|
|
LDL Direct |
60 |
<130 mg/dL |
|
Poc Creatinine Result |
Value |
Ref Range |
|
Creatininer, POO |
1.0 |
0.7- 1.3 mg/dL |
|
ECG 12 lead Result |
Value |
Ref Range |
|
INTERPRETATION TEXT |
Not Confirmed |
|
Table 1 Laboratory work up at emergency room (ER) of a local hospital
Blood supply to the retina is complex and vital. The inner retina receives its blood from the central retinal artery, which originates from the ophthalmic artery, the first branch of the internal carotid artery after it exits the cavernous sinus. The central retinal artery follows a unique path, piercing the optic nerve sheath obliquely, traveling briefly between the meningeal sheath and optic nerve, entering the nerve substance, and emerging at the optic nerve head to branch into terminal arterioles supplying the retina. Interestingly, about 20% of individuals have an accessory branch from the posterior ciliary circulation called the cilioretinal artery, which supplies the macula and can potentially preserve central vision in cases of central retinal artery occlusion.8 The outer retina and choroid, on the other hand, receive their blood supply from the posterior ciliary arteries, also branches of the ophthalmic artery.9,10 As neural tissue, the retina is highly sensitive to ischemia. While it was previously thought that retinal cells die within 15 minutes of blood flow interruption, recent experimental and clinical evidence suggests a longer window of viability, potentially up to 240 minutes.11 Two possible reasons for this extended tolerance: passive diffusion of oxygen from the adjacent outer retina and choroid; and passive perfusion from radial branches of central retinal artery that collateralize with the pial arteries in the optic nerve shealth.9
Non-arteritic central retinal artery occlusion (CRAO) is a severe form of acute ischemic stroke affecting the eye, leading to significant visual and functional impairment. The outlook for individuals with CRAO is generally unfavorable. Fewer than 20% of patients regain a functional visual acuity better than 20/200 in the affected eye because of a lack of timely and effectively reperfusion of the retina.12
The typical presentation of central retinal artery occlusion (CRAO) involves a sudden, unilateral painless loss of vision, and a relative afferent pupillary defect. Its hallmarks in acute setting include optic nerve head swelling, retinal whitening and the appearance of a cherry-red spot at the fovea.7 These changes reflect the ischemic swelling of inner retina, and the lacks an overlying retinal layer at the fovea that permits the underlying choroidal vasculature visible. Over the course of a month, the initial retinal swelling gradually subsides, leading to thinning of the inner retinal layers, attenuated vessels, retinal pigment epithelial mottling and pale optic disc.7
The diagnosis of CRAO is relatively simple because of the severe sudden loss vision and the presence of retinal whitening with cherry spot. However, a few differential diagnoses are warranted: ophthalmic artery occlusion and branch retinal artery occlusion (BRAO). Ophthalmic artery occlusion blocks both choroidal and retinal perfusion. Vision is often no light perception, and there is no cherry red spot visible from choroid. Conversely, BRAO is limited to sectoral whitening in the path of the affected arterial branch. Additionally, cherry red spots can also be seen in Commotio retinae (whitening of the retina following blunt trauma to the eye), Tay-Sachs (a rare genetic disorder leading to toxic accumulation of fatty substances), and Niemann–Pick diseases (a rare genetic disorder leading to poor metabolism of lipids).13
CRAO demands urgent medical attention and rapid intervention. Swift diagnosis and treatment are crucial, as the chances of preventing irreversible damage increase with faster response times. The primary objective in CRAO management is to quickly dislodge the obstructing embolus and promptly restore retinal blood flow and preserve retinal cell function. Time is retina because the critical window to rescue from irreversible damages is within 240min from symptom onset. Unfortunately, majority of cases showed to urgent care setting or the eye clinic after the critical time window when not much can be done to revive the affected retina.
Over the decades, various treatment attempts to induce vasodilation or reduce IOP in hoping to dislodge the embolus have been performed without significant success; hence, current professional guidelines for CRAO management do not recommend them.12 These include anterior chamber paracentesis, ocular massage, intraocular pressure-lowering agents, sublingual isosorbide dinitrate, systemic b-blockade, carbogen therapy (95% O2, 5% CO2), and paper bag breathing. Other treatment modalities have been investigated and are discussed in more details in a recent review Venkatesh and colleagues.14 They are neodymium: yttrium‑aluminium‑garnet (Nd: YAG) laser arteriotomy and embolectomy, pars plana vitrectomy (PPV), PPV plus endovascular surgery, local intra‑arterial thrombolysis (IAT), hyperbaric oxygen therapy (HBOT) (Table 2). Although an effective treatment for acute CRAO remains to be found, similar approach to treatment of stroke, by deploying clot busting agents within the critical < 6 h window, appears promising. Presently, a few clinical trials involving intravenous thrombolytic agents are being conducted with results anticipated in the next few years.15
|
Treatment rationale |
Procedures |
|
Reduce IOP to enhance perfusion throughout the optic nerve head |
*Anterior chamber paracentesis |
|
*IV acetazolamide |
|
|
*Pars plana vitrectomy |
|
|
Vasodilate to increase arterial blood flow |
*Inhaled carbogen (95% O2, 5% CO2) |
|
*Sublingual isosorbide dinitrate |
|
|
*Hyperbaric oxygen (2-atmosphere absolutes) |
|
|
*Pentoxyphilline |
|
|
To lyse or dislodge the emboli |
*Ocular massage |
|
*Transluminal Nd:YAG laser |
|
|
* Pars plana vitrectomy |
|
|
*IV Thrombolytic agents (Alteplase, Tenecteplase) |
Table 2 Acute CRAO management rationales and procedures
This case report is rare because the patient suffered both from an acute CRAO and PED after a prolonged coughing episode. His ONH was swollen with indistinct margin, and the posterior pole was pale with a typical fovea cherry spot (Figure 1B). Extensive swelling of inner retina and PED extending from the ONH were apparent on OCT imaging (Figure 2B). When he showed up to the UCC the next day, it had already passed the critical window of 6 hours so not much could be done ocularly to reverse the retinal damages in the left eye. The patient was referred to a local retinal specialist who then sent the patient to the emergency department for a more extensive stroke work up at a local hospital emergency department. There, he was found to have left proximal ICA stenosis, chronic atrial fibrillation, interstitial lung disease, chronic GERD, and severe shoulder arthritis. Then, he was discharged in stable condition and scheduled to have continued care with retinal specialist, PCP, pulmonologist and cardiologist.
A few lessons from this case are
|
Etiology |
Investigation |
|
Common risk factors |
Family history of cardiovascular (TIA, angina), cerebrovascular diseases. |
|
Diabetes mellitus |
|
|
Dyslipidemia |
|
|
Vulvular heart disease |
|
|
Smoking |
|
|
Vascular risk factors |
Blood pressure |
|
Fasting blood glucose level |
|
|
Fasting cholesterol levels |
|
|
Embolic source |
Duplex carotid ultrasound |
|
Echocardiogram |
|
|
Young (<50 yo) without vascular risk factors |
Hypercoagulable screen (protein C&S, factor V Leiden, anti-phospholipid antibody) |
|
Vascultitic screen (ANA, ENA, ANCA, ACE) |
|
|
Myeloproliferative or sickle cell (blood film) |
Table 3 A handy checklist on common risk factors and investigations for acute CRAO management
Although it is rare to encounter ocular emergencies in the eye clinic, but it can be in your chair; sometimes, two urgent conditions can show up simultaneously. The primary role of the eye care provider is to diagnose the acute CRAO and activate the local team and resources available to the patient. Therefore, clinicians should be prepared with a handy reference what to do, and which team to activate to achieve optimal co-management to preserve vision and save life.
Thanks the UCC provider and ophthalmic technicians at MG-VAMC for their assistance in patient care.
This case report is original, and the authors have no commercial interest in the subject of study. The authors do not have any financial conflicts of interest and no funding agency is involved in this case report.
None.

©2025 Koh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.