Advances in
eISSN: 2377-4290


Research Article Volume 15 Issue 2
1Graduate/Degree in Medicine, Ophthalmology Department, Hospital clínico San Borja Arriarán, Chile
2Graduate/Degree in Medicine, Ophthalmology Department, Hospital Universitario Nuestra Señora de la Candelaria, Spain
3Doctor of Medicine, Ophthalmology Department, Hospital Universitario Nuestra Señora de la Candelaria, Spain
4Doctor of Medicine, Ophthalmology Department, Hospital Clínico Felix Bulnes Cerda, Chile
Correspondence: Perez Gonzalez M, Doctor of Medicine, Ophthalmology Department, Hospital Clínico Felix Bulnes Cerda, Santiago, Chile
Received: April 23, 2025 | Published: May 19, 2025
Citation: Arshavin BP, Peralta QG, González AR, et al. Biomarker analysis in diabetic macular oedema: Baseline and one-year follow-up characteristics in real clinical practice. Adv Ophthalmol Vis Syst. 2025;15(2):31-34. DOI: 10.15406/aovs.2025.15.00481
Objective: To describe the baseline characteristics of patients with diabetic macular oedema (DMO), both clinical and OCT biomarkers using AI and compare them at one-year follow-up.
Methods: A descriptive cross-sectional and retrospective study was carried out on diabetic patients with a new diagnosis of DME and naive to treatment who consecutively attended our center over a period of two years. These patients were followed up during the year following their inclusion.
Results: The study included 72 patients diagnosed with DME. No significant differences were found in age, visual acuity (VA) and glycosylated hemoglobin between sexes, except for dyslipidemia, which was more prevalent in men. At one year follow-up, there was a significant reduction in intraretinal fluid (IRF), but not in subretinal fluid (SRF) or pigment epithelial detachment (PED). In addition, there was a significant increase in the prevalence of epiretinal membrane (ERM) and geographic atrophy (GA).
Conclusion: The study highlights a significant reduction of intraretinal fluid in patients with DME after one year of follow-up, but also an increase in the prevalence of epiretinal membrane and geographic atrophy. These results emphasize the importance of using advanced tools such as OCT with AI for better assessment and prognosis of DME in real clinical practice.
Keywords: diabetic macular oedema, tomographic biomarkers, artificial intelligence in ophthalmology
Diabetes mellitus (DM) is a chronic metabolic disease characterized by sustained hyperglycemia, caused by defects in insulin secretion and/or action. This condition affects a significant proportion of the general population and is a risk factor for multiple microvascular and macrovascular complications. According to reports from the International Diabetes Federation (IDF) around 537 million people had diabetes in 2021, representing a global prevalence of 10.5% and this number is projected to increase in the coming years.1 Among the ocular microvascular complications, diabetic retinopathy and diabetic macular oedema (DME) represent one of the main causes of vision loss, the latter with a prevalence of 4-8% in patients with type 1 DM and 1-12% in patients with type 2 DM.2,3
DME corresponds to the accumulation of extracellular fluid at the retinal macular level due to the breakdown of the blood-retinal barrier (BRB), mediated by an increase in vascular permeability. This fluid accumulation correlates with retinal thickening detectable by optical coherence tomography (OCT).4 OCT with artificial intelligence (AI) analysis has emerged as a useful tool in the assessment and monitoring of DME, providing key quantitative and qualitative biomarkers that reflect retinal morphology.5 Among the most important OCT-detectable biomarkers are central macular thickness (CMT), the presence of intraretinal cysts, retinal pigment epithelium detachment (DEP) and hyperreflective spots (HF). An increased CMT is indicative of macular oedema and correlates with decreased VA. Intraretinal cysts reflect extracellular fluid accumulation due to BHR dysfunction, while DEP and HF may indicate underlying inflammatory or ischemic processes. In addition, changes in the reflectivity of retinal layers, such as disruption of the ellipsoid zone, provide information on structural damage and residual visual function.6,7 This approach is improving the interpretation of OCT-derived biomarkers in DME, significantly improving the accuracy and efficiency of disease diagnosis and monitoring.
Our aim is to study patients with DME from a profile of their tomographic biomarkers and see how they vary in the follow-up of their pathology and through the multiple treatments they undergo throughout their disease. This approach allows us to understand the baseline reality of our patients and the impact of their disease over time in relation to their retinal anatomy and VA. For this purpose, we will describe the baseline characteristics of OCT biomarkers with the help of AI and compare them at one-year follow-up.
A descriptive cross-sectional and retrospective study was conducted on adult diabetic patients with a new diagnosis of DME and naive to treatment who consecutively attended our center (Nuestra Señora de Candelaria University Hospital, Santa Cruz de Tenerife) from January to December 2019. These patients were followed up for one year after their inclusion date. Patients with a history of previous ocular surgery, coexisting ocular diseases and a history of previous ocular trauma were excluded.
The variables of age, sex, comorbidities, glycosylated hemoglobin (HbA1c) levels and VA were obtained from the electronic medical record. All data were obtained anonymously and the ethical guidelines of the declaration of Helsinki were followed.
Diabetic macular oedema (DME) was diagnosed based on the presence of intraretinal cysts and/or subretinal fluid on spectral-domain OCT, along with central retinal thickness greater than 300 µm. All scans were evaluated by retina specialists to exclude other causes of macular oedema, such as pseudophakic cystoid macular oedema, inflammatory disease, or vein occlusion. Only patients with DME secondary to diabetic retinopathy were included.
OCT scans were performed with the Heidelberg tomograph (Spectralis, Heidelberg, Germany) and analyzed using the AI Discovery 4.4 platform (RetinAI, Switzerland) for quantification of retinal biomarkers, focusing on the central OCT scan areas of 1 mm, 6 mm and total macular cube (Figure 1). Data included tomographic variables such as subretinal fluid (SRF), intraretinal fluid (IRF) and pigment epithelial detachment (PED), measured in nanoliters. Biomarkers included SRF, IRF, HF, drusen, reticular pseudodrusen (RPD), epiretinal membrane (ERM), geographic atrophy (GA) and outer retinal atrophy (ORA). A biomarker was considered to be present if its probability of being found by AI was equal to or greater than 90%. Unless otherwise specified, quantitative thickness values correspond to the central 1-mm ETDRS subfield.
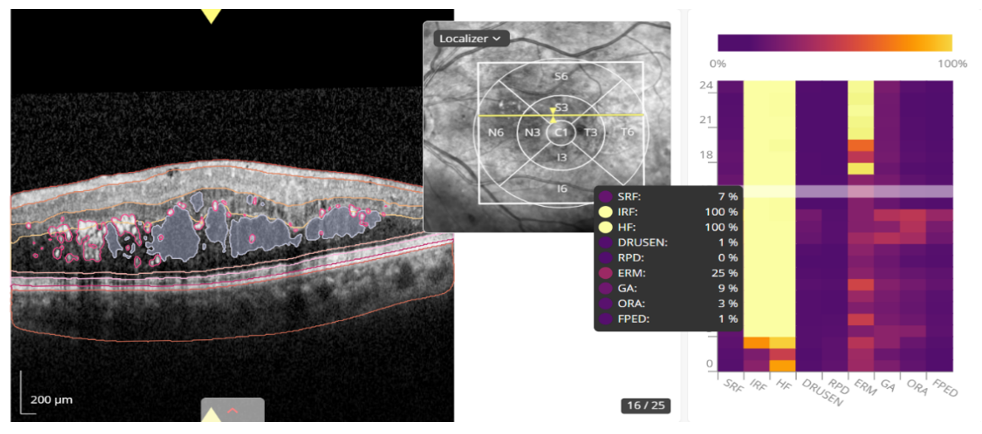
Figure 1 OCT analysis interface using AI (Discovery 4.4) of a patient with DMO. On the right, the different probabilities of presence of each biomarker given by the AI system are shown.
All AI-detected features, including ERM, GA, and BHR disruption, were independently reviewed by two masked retina specialists. Disagreements were resolved by consensus.
A total of 72 patients with DME were recruited and the same number of eyes analyzed. Table 1 summarizes their demographic characteristics of sex distribution, age, baseline HbA1c values, VA in Log MAR and prevalence of chronic arterial hypertension (AHT), dyslipidemia and smoking. Normality tests showed an absence of normal distribution in the variables, so the Mann-Whitney U test was performed to compare them, finding only significant differences in the prevalence of dyslipidemia, with a higher proportion in men (p< 0.05). Best-corrected visual acuity (BCVA) after treatment was recorded at the 12-month follow-up visit.
|
Population |
Total patients |
Men |
Women |
|
No. of participants |
72 (100%) |
53 (74%) |
19 (26%) |
|
Average age (years) |
64 (SD 12.6) |
67 (SD 10.7) |
56 (SD 14.3) |
|
Mean HbA1c (%) |
7.7 (SD 2.1) |
8.0 (SD 2.0) |
6.8 (SD 2.3) |
|
Visual Acuity (LogMAR) |
0.5 |
0.52 |
0.45 |
|
Arterial hypertension |
52 (72%) |
40 (75%) |
12 (63%) |
|
Dyslipidaemia |
60 (83%) |
47 (89%) |
13 (68%) |
|
Smoking |
18 (25%) |
12 (23%) |
6 (33%) |
Table 1 Variables and their distribution according to sex. Standard deviations (SD) are shown for age and HbA1c. Percentage of total is shown in brackets (* p < 0.05)
Mean IRF, SRF and PED were calculated at the baseline visit and compared with their values at one-year follow-up. Since the sample did not follow a normal distribution, analysis was performed using the Wilcoxon test for paired samples (Table 2). There is a significant decrease in IRF at one year follow-up, but not in SRF or PED.
|
Parameter |
Baseline average (µL) |
1-Year average (µL) |
p-value |
|
IRF* IRF* IRF* IRF* IRF* IRF* IRF* IRF* IRF |
505 (DE 632.9) |
280 (DE 635.1) |
<0.05 |
|
SRF |
25 (SD 72.4) |
29 (OUT OF 126) |
0.49 |
|
PED |
1.9 (SD 6.3) |
3.3 (SD 18.5) |
0.87 |
Table 2 OCT biomarkers detected by AI at baseline and at 12-month follow-up. Averages in microliters (μL) of baseline and one-year follow-up values of SRF, IRF and PED. Wilcoxon p-statistic values are shown (* p < 0.05)
IRF, intraretinal fluid; SRF, subretinal fluid; PED, pigment epithelium detachment
Table 3 shows the percentage prevalence of the different biomarkers both at baseline and at one-year follow-up. A biomarker is considered present if the probability given by the AI is greater than 90%. Those that showed statistically significant changes at one year follow-up were the increased presence of MRD and GA (p<0.05).
|
Biomarker |
Baseline (n: 72) |
1 Year (n: 72) |
|
SRF |
28% |
19% |
|
IRF |
99% |
94% |
|
HF |
99% |
93% |
|
Drusen |
25% |
25% |
|
DPO |
7% |
7% |
|
ERM* ERM* ERM* ERM* ERM* ERM* ERM* ERM* ERM |
43% |
54% |
|
GA |
26% |
42% |
|
ORA |
12% |
14% |
|
FPED |
24% |
12% |
Table 3 Percentages represent the proportion of patients within each biomarker category showing presence or absence at baseline and at one-year follow-up. (* p < 0.05)
Three different drugs were used for intravitreal treatment: Ranibizumab (Lucentis), Aflibercept (Eylea) and Dexamethasone (Ozurdex). Lucentis was used in 69 patients (95%), Eylea in 3 patients (4%) and Ozurdex in 2 patients (3%). This percentage distribution is due to the fact that there was 1 patient who switched from Lucentis to Ozurdex and 1 patient who switched from Lucentis to Eylea during the 1-year follow-up. During the year of treatment an average of 3.5 injections of Lucentis (SD 1.3), 3.0 injections of Eylea (SD 2.0) and 1.0 injections of Ozurdex (SD 0) were used. There was only 1 patient in whom Ozurdex was started as first-line treatment.
In our study we found a higher prevalence of men with higher average age and HbA1c levels than women, although none of these differences reached statistical significance. Within the gender differences in diabetes it has been found that men tend to have higher Hb1Ac levels for both biological and socio-cultural reasons.8 The relationship between Hb1Ac levels and ocular complications of DM has been well documented for quite some time. Both the United Kingdom Prospective Diabetes Study (UKPDS)9 and the Diabetes Control and Complication Trial (DCCT),10 two of the largest studies on this topic, demonstrated the directly proportional relationship between Hb1ac levels and the increased prevalence of microvascular complications, including DME, as well as lower blood glucose levels reducing the overall incidence of microvascular complications in diabetic patients. Thus, the higher representation of men in the sample could be related to their higher HbA1c levels. The proportions of comorbidities of HTN, dyslipidemia and smoking observed are similar to those observed in similar studies;11 in the case of HTN and diabetic retinopathy (DR), the association is not very clear. There are studies showing that there is no greater exacerbation of these complications with higher blood pressure levels.12 What is clear from studies such as the UKPDS9 is that strict control of blood pressure levels decreases the prevalence of DR.
In DME the characteristic OCT changes are an increase in CMT associated with macular oedema mainly involving the inner nuclear layer (INL) and the outer plexiform layer (OPL). As the duration of oedema is prolonged, fluid spaces begin to appear which may coalesce into large intraretinal cysts.13 Both IRF and SRF are characteristic of DME, with IRF present in almost all patients at diagnosis and SRF in less than half of the cases.14 This is evidenced by the fact that at the baseline visit the main fluid in terms of volume (μL) found is IRF, with the μL values of SRF and PED being quite minor in comparison, thus the large significant change at one year of treatment correlates with the decrease in the IRF compartment showing a good response to treatment.15 Their biomarkers also show a decrease at one year of treatment although it is not significant. This is due to the fact that at the slightest presence of one of these biomarkers, the AI considers them to be present, showing the importance of evaluating biomarkers and their volumes as a whole. With regard to biomarkers, the main findings were an increase in the presence of MRD and GA at one-year follow-up compared to the baseline visit. Currently the treatment of choice for DME is anti-VEGF drugs. Although their functional benefit on VA and structural benefit on OCT has been extensively studied, an increase in retinal atrophy and inner retinal changes after repeated use has also been reported, mainly in the context of AMD.16,17 Karst et al. studied the incidence of geographic atrophy in patients with DME and found that 4% of patients developed geographic atrophy at four years of follow-up.18 They studied atrophy through retinal thickness in microns, unlike us who measured it in the presence of biomarkers, which may explain the differences found. It would probably be useful in the future to study geographic atrophy in these patients by measuring the retinal area affected. Geographic atrophy is relevant as the relationship between increased VA loss and damage to the outer retina has been known for some time. Studies show a strong correlation between damage to external structures, mainly those related to photoreceptors, and decreased VA.19,20 This same study does not show the same correlation with CMT. This demonstrates the importance of studying biomarkers related to the outer retina as they compromise the visual prognosis of patients. A key limitation of this study is that not all AI-based OCT biomarkers were validated by human graders, which may introduce classification bias or overestimation of some features.
The increased prevalence of MRD may be related to its inflammatory etiology. Several studies show an increase of inflammatory cytokines in the vitreous cavity in patients with DR which has been associated with the increased prevalence of MRD in these patients.21 This shows the importance of vitreoretinal interface assessment in patients with DME as the presence of MRD may be an added factor to the decrease in VA, although it does not seem to influence the response to treatment. Karaküçük et al. evaluated VA and OCT improvement following monthly loading dose with ranibizumab without finding significant differences between patients with and without MRD.21,22
The use of AI in ophthalmology has brought many benefits in clinical practice. Currently there are tools such as algorithms for the interpretation of diabetic retinopathy such as the LuxIA algorithm23 or others that also include the identification of pathologies such as glaucoma and AMD such as RetinaLyze. Several studies have shown similar performance of these AIs when compared to interpretation by a trained ophthalmologist.24 In the case of AIs that analyze OCT data such as Discovery, they are often based on different Deep Learning (DL) methods including generative antagonistic networks (GAN) that are able to automatically segment the different retinal layers and quantify retinal fluids.25 The correct segmentation of retinal layers is essential for the detection of alterations such as disorganization of the inner layers (DRIL), photoreceptor integrity and the presence of geographic atrophy. While the implementation of these new algorithms can be beneficial, it also has certain limitations. By requiring multiple data inputs, certain demographic groups or rare disease characteristics may be under-represented. Concerns have also been raised about the privacy of the data obtained and the accountability of making decisions based on an algorithm over human judgement, which is currently under regulation in several countries.
The biomarker study in DME reveals that, after one year of follow-up, patients show a significant reduction in IRF, suggesting a favorable response to treatment and a possible stabilization of BHR. However, an increase in the prevalence of MRE and GA, indicators of retinal structural deterioration that could negatively affect long-term VA, is also observed. These findings underline the importance of using advanced tools such as AI-assisted OCT, which allows more accurate detection and detailed quantification of retinal biomarkers, thus improving the ability to monitor subtle changes in retinal anatomy and optimize treatment strategies. The implementation of AI in OCT assessment not only facilitates faster and more accurate diagnosis, but also contributes to the personalization of therapeutic plans, tailoring them to the specific needs of each patient and improving clinical outcomes in daily practice.
None.
The authors declare that there are no conflicts of interest.
None.

©2025 Arshavin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.